Intralesional Path to Cancer Drug Approval
Fill Factor: the ratio of PV-10 volume to tumor volume to deliver a constant dose of rose bengal sodium per quantity of tumor
Multiple investigational intralesional (IL) agents have been tested across a range of solid tumor cancer types, in addition to synthetic small molecule PV-10. While the majority of these IL agents have been oncolytic viruses (OVs), several were biologics.
The only IL agent to garner FDA approval to date is Imlygic®, an OV based on a modified herpes simplex virus (HSV) type 1 and designed to selectively replicate in and lyse tumor cells upon IL injection, with secondary potential to promote regional and systemic anti-tumor immunity.[1] The dosing scheme for Imlygic is predicated on administering sufficient OV into tumor tissue to promote destruction of injected tumors, an approach that appears to be based on a number of predicate IL biologic agents.
Imlygic and its key predicate agents, as well as several agents developed in parallel to it, are reviewed in an overview of strategies utilized during clinical development.
A. Predicate Biologic Agents Cited in Imlygic’s Development
a. BCG
The earliest IL agent to undergo detailed testing in cutaneous melanoma was Bacillus Calmette-Guerin (BCG), led by reports of “objective improvement” in five of 15 patients with Stage III disease receiving single IL injections (3x106 bacilli, volume of injectate not reported) every two weeks for an initial five cycles, with treatment continued if improvement was noted until complete response or progression.[2] This was followed by a small randomized study in patients with in-transit melanoma (ITM), where BCG was compared to dinitrochlorobenzene (DNCB).[3] BCG in saline was injected into up to 25 skin lesions (0.1 mL/lesion containing 5x107 bacilli), which was repeated every 4-6 weeks (using 0.1 mL/lesion at 5x106 bacilli) until all lesions were injected, with a few lesions injected twice; DNCB was administered in similar fashion, with injection of 0.1 mL of an acetone formulation into intradermal lesions and small (<1 cm) subcutaneous lesions, with 0.1-0.2 mL administered to larger subcutaneous nodules. Both agents exhibited good local effect (ca. 90% local response) but there was no evidence of systemic benefit. However, BCG was also associated with an unacceptable risk of significant toxicity, with three of 25 patients experiencing disseminated intravascular coagulation (DIC), including one near fatality, while there were minimal adverse experiences in 51 patients receiving DNCB. These observations led to loss of interest in BCG for melanoma although BCG has become a standard immunotherapy for superficial bladder cancer administered via intravesical instillation.[4]
b. Allovectin-7
Allovectin-7 (velimogene aliplasmid, an immune-stimulating biologic construct composed of a bicistronic plasmid encoding human leukocyte antigen-B7 and beta-2 microglobulin plasmid formulated with cationic lipids intended to recruit macrophages and T cells) was studied in metastatic melanoma in an open-label Phase 2 study (77 patients enrolled at 16 centers).[5] Patients were given six once-weekly intralesional injections of 10 µg Allovectin-7 (volume not specified) followed by four weeks of observation for up to three treatment cycles. The investigational treatment exhibited minimal adverse events, with modest activity (9% objective response, 4.8 months median duration of response), supporting further testing at higher dose.
A second Phase 2 dose-escalation study evaluated Allovectin-7 in 133 patients with Stage III/IV metastatic melanoma recurrent or unresponsive to prior therapy.[6] Patients received six weekly intralesional injections followed by three weeks of observation and evaluation; patients with stable or responding disease could receive further cycles of study treatment. The number of injectable lesions was determined by the investigator, and could be cutaneous, subcutaneous, or nodal lesions ≥1 cm2 and ≤25 cm2. It appears that 1 mL of investigational agent was administered per lesion. A 3 x 3 dose escalation scheme was employed, with cohorts receiving 0.5, 1.0 or 2.0 mg Allovectin-7; based on initial safety observations, remaining patients received 2.0 mg in an efficacy cohort. Patients in the efficacy cohort with multiple injectable lesions could have up to 5 lesions injected in a treatment cycle (i.e., 2 mg Allovectin-7 divided equally among all injected lesions). Thus, it does not appear that a consistent intralesional dose (volume or mg Allovectin-7) was employed. An objective response was observed in 12% of patients, along with acceptable toxicity.
In a pivotal Phase 3 study, Allovectin-7 was tested vs chemotherapy in 390 Stage III/IV melanoma patients (randomized 2:1 against dacarbazine or temozolomide), with administration of 2 mg to a single lesion weekly for six weeks, repeated after each eighth week; the primary endpoint was response rate at 24 weeks aher randomization.[7] An objective response was achieved in 4.6% of patients receiving Allovectin-7, vs 12.3% receiving comparator, while overall survival was 18.8 months vs 21.3 months. Considering these results, no further studies of Allovectin-7 were conducted in melanoma.[8] Further details on dosing were not published, but study participants were required to have at least one injectable lesion 1 x 1 cm or greater in size.
c. HSV1716
Phase 1 testing of the IL oncolytic virus HSV1716 is cited as predicate work in reports on initial development of Imlygic. Rampling et al. evaluated toxicity of HSV1716, a Phase 1 study administering the agent intralesionally to relapsed glioma.[9] Patients received a single treatment with one of three doses of agent (103, 104, or 105 pfu/mL, N = 3 patients per dose) via stereotactic injection into a single brain tumor (8.6 to 129 cm3 tumor volume) at 6-10 radial locations within the tumor; a total of 1 mL of agent was administered. Hence, no consistent IL dose (volume or pfu of OV) was employed. No encephalitis, adverse clinical symptoms, or reactivation of latent HSV were observed.
d. CV706
CV706 is also cited as predicate work in reports on initial development of Imlygic. Non-clinical testing of CV706 (formerly known as CN706), a replication-competent PSA-selective OV based on an adenovirus planorm, was conducted in nude mice with implanted LNCaP human prostate cancer tumors.[10] Solitary flank tumors were injected with 0.1 mL of virus suspension (5x108 pfu) when they reached approximately 1 cm diameter (i.e., ca. 0.1 mL / 0.5 cm3 tumor); complete regression was noted in 5 of 10 tumors.
Subsequent Phase 1 testing of CV706 was conducted in patients with locally recurrent prostate cancer following radiation therapy.[11] Twenty patients were divided into 5 dose cohorts, receiving 1x1011 to 1x1013 viral particles delivered via transperineal injection using a modified prostate brachytherapy technique. CV706 was administered as 0.1 mL injections at 20-80 locations (“intraprostatic viral deposits”) using 10-40 needles. Dosing was based on a “viral dosimetric” algorithm derived from non-clinical experience to determine optimal sites for injection to achieve homogeneous coverage of the prostate, where each injection was presumed to “result in ~1 mL of tumor killing.” The primary endpoint was determination of treatment-related toxicity, and the investigational treatment was found to be safe, with no irreversible grade 3 or any grade 4 toxicity. Patients in the highest two dose groups achieved a ≥50% reduction in serum PSA. Further clinical development does not appear to have been done.
e. G207
An HSV-based OV, G207, is also cited as predicate work in reports on initial development of Imlygic. This virus has been modified to selectively target glioblastoma multiforme (GBM) while minimizing infectivity of normal tissue.
An initial Phase 1 dose-escalation study, G207 was administered via stereotactic injection to single treatment-refractory cerebral malignant glial tumors >1 cm diameter in 21 adult patients.[12] Pre-treatment and post-treatment viable tumor volumes were estimated using an MRI eigenvalue algorithm to determine enhancing tumor volume only, which showed a mean volume of 39 cm3 at baseline. Patients in the initial five cohorts x three patients received 1x106 to 3x108 pfu (0.1 mL administered to a single loci); those in the sixth cohort received 1x109 pfu (0.3 mL administered to a single loci); and those in the final cohort received 3x109 pfu (0.2 mL administered to each of five loci, 1.0 mL total volume). No toxicity or serious adverse events were attributed to G207, while there was some evidence of anti-tumor activity.
In an additional Phase 1 dose-escalation study, G207 was administered to children and adolescents with recurrent or progressive supratentorial brain tumors.[13] A 3+3 design with four dose cohorts administered 107 or 108 pfu (total volume 2.4 mL) over a period of six hours via 3-4 stereotactically implanted catheters (patients in cohorts 3 and 4 received 5 Gy radiation to the tumor mass 24 hours after G207 administration). At baseline, treated tumors ranged from 2.4 to 6.7 cm in longest diameter (median 3.4 cm). No DLTs aNributed to G207 were observed, with radiographic, neuropathological, or clinical responses observed in 11 of 12 patients.
In a Phase 1b study of G207 in 6 patients with recurrent GBM, a catheter with a solid tip and 16 openings in a circumferential pattern beginning 1 cm from the tip and extending for approximately 2 cm was placed in enhancing tumor; once placed, 1 mL of agent (1.5x108 pfu) was infused over a 10-minute interval, followed by an additional 0.8 mL of saline to clear the catheter. [14] After administration, the catheter was sealed, and the tumor was subsequently resected 2-5 days later. Immediately after resection, the resected tumor bed was injected at multiple locations with G207 (1x109 pfu, 6 mL total volume, administered in approximately equal portions at a depth of 1 cm space approximately 1 cm apart using a 28G needle). Minimal toxicity was observed with radiologic and neuropathologic evidence suggestive of antitumor activity.
In another Phase 1 trial in nine patients with progressive, recurrent GBM, stereotactic IL administration was used to inoculate multiple sites of the enhancing margin, followed one day later by focal treatment with 5 Gy radiation (6 MV linear accelerator).[15] The investigational agent (0.2 mL G207 at 1x109 pfu) was injected into 5 different areas “over 2 minutes to avoid possible reflux.” Treatment was well tolerated, with modest evidence of clinical response to the single-dose OV therapy.
f. ONYX-015
A genetically modified attenuated adenovirus, ONYX-015, is also cited as predicate work in reports on initial development of Imlygic. This virus has been modified to selectively replicate and kill cells harboring p53 mutations, including head and neck, gastrointestinal, and pancreatic tumors, and is purported to leave normal cells unaffected.
Initial preclinical testing of ONXY-015 (also known as dl1520) was conducted in tumor xenograph models (C33A human cervical carcinoma and U87 human glioblastoma tumors implanted in flanks of nude mice);[16] tumors were injected every other day for three doses beginning when they reached palpable size (mean tumor volume 150 µL). In a second series of experiments, implanted flank tumors were injected when they reached 80 µL, receiving 108 pfu suspended in 60 µL of vehicle for five consecutive days, with dose divided equally into each tumor quadrant. C33A tumors exhibited significant regression.
The agent underwent further preclinical testing using a several human xenograph tumor models (HLaC laryngeal carcinoma, in addition to C33A cervical carcinoma and U87 glioblastoma) in nude mice.[17] Implanted flank tumors were injected daily for five days using 108 pfu of ONYX-015 suspended in 60 µL of vehicle starting when they reached a size of 65-80 µL (5- to 8-mm maximum diameter). Vehicle-only and inactivated virus injection controls were also tested. Improved survival was observed in HLaC and C33A mice but not in U87 mice.[18]
A Phase 1 trial of ONYX-015 evaluated safety and biological activity of single IL injections in 22 patients with recurrent head and neck cancer.[19] Tumors were injected based on estimated volume, with dilution of ONXY-015 adjusted so that total injection volume equated to 30% of estimated tumor volume; tumor was mapped into 1 cm2 areas, with equal volumes injected into each portion. Patients could receive retreatment at four-week intervals for up to five cycles (64ti received a single cycle, none received 5 cycles). Injected tumors ranged from 2.2 to 20 cm2, with a median of 11.8 cm2 (i.e., ca. 1.5 to 4.5 cm diameter, median ca. 3.4 cm). Six doseescalation cohorts received 107 to 1011 pfu, with no dose limiting toxicity reached. While no objective tumor responses were observed, MRI scans “were suggestive of tumor necrosis at the site of viral injection in five patients”. Additionally, three patients had satellite tumors injected with diluent alone, all of which progressed. The authors noted that “two patients did experience mild pain on injection…related to the volume of the injected solution” that resolved spontaneously within 1 hour.
In another Phase 1 dose-escalation trial, ONYX-015 was administered every four weeks to primary pancreatic tumors intraoperatively (N = 1 patient) or under CT guidance (N = 22 patients) using a 22-ga needle.[20] Diluted virus (108 to 1011 pfu) was delivered at 10% to 20% of estimated tumor volume, with number of injections dependent on size of tumor: ≤3 cm received one central injection; >3 to≤6 cm received two injections to the respective centers of the tumor halves; >6 cm received 3 injections. The volume of injected agent was distributed equally among injection sites. All dose levels were tolerated but no objective responses were observed nor was there evidence of viral replication.
In a Phase 1/2 trial, ONYX-015 was administered to 21 patients with locally advanced or metastatic pancreatic adenocarcinoma (mPDAC), but with no or minimal liver metastases; patients received 2x1010 or 2x1011 pfu in a 3+3 dose escalation scheme, with an additional 15 patients receiving the higher dose; ONYX-15 was administered to the primary tumor weekly under ultrasound guidance over 8 weeks, with gemcitabine given concurrently with the final four treatments.[21] The volume of injected virus was adjusted to comprise one-tenth of tumor volume. The authors concluded that the treatments were generally well tolerated with partial or minor regression of the injected tumor observed in four subjects (19% of subjects).
In a Phase 2 trial, ONYX-015 was tested in patients with recurrent/relapsed SCC of the head and neck (HNSCC).[22] The agent was administered intralesionally to a single identified tumor on five consecutive days repeated every three weeks (30 patients in a standard treatment cohort) or twice daily on 14 consecutive days repeated every three weeks (10 patients in a hyperfractionated treatment cohort) with a total of 533 injections administered. The agent was administered at 1010 pfu into each of five equally spaced, equally sized tumor quadrants, with concentration adjusted so that total injection volume equated to 30% of estimated tumor volume. Partial or complete response was observed in 14% of patients in the standard treatment cohort, while 41ti exhibited stable disease; in the hyper-fractionated cohort, 10% of patients achieved complete response with another 62% achieving stable disease. In addition, acceptable safety was demonstrated.
B. Imlygic Pre-Clinical Development and Testing
Imlygic (also known as talimogene laherparepvec, JS1/ICP34.5-/ICP47-/GM-CSF, OncoVEXGM-CSF, or T-Vec) was tested in vitro then in vivo in mouse xenograph models using human tumor cell lines.[23] Flank tumors were injected three times (2-3 days apart) with an intermediate test article (an OV that did not contain all the coding features of T-Vec): implanted HT-29 colon, Fadu carcinoma, and U87MG glioma tumors were injected with 50 µL of agent when they reached a size of 5 mm diameter (i.e., 50 µL injected into approximately 65 µL of tumor volume) and tumor growth followed for several weeks after final injection. A bilateral flank model of A20 lymphoma was employed to evaluate T-Vec injected into one flank tumor (5 mm diameter, 50 µL injected three times every other day) at 106, 107, and 108 plaque forming units (pfu) / mL with growth followed thereafter. Because T-Vec is built on an HSV backbone, the role of prior HSV infection was studied and found to be irrelevant.
C. Imlygic’s Phase 1 Study
Imlygic was studied in a dose escalation study in patients with cutaneous or subcutaneous solid tumors who had failed prior therapy.[24] Thirteen patients were enrolled in a single-dose group and received Imlygic at 106, 107, or 108 pfu/mL (N = 4, N = 5, and N = 4, respectively), while another 17 patients were in a multidose group testing a number of dose regimens (these received an initial dose of 106 pfu/mL followed by 107 or 108 pfu/mL, N = 3 and N = 14, respectively). High dose treatments (108 pfu/mL) were limited to patients that were seropositive for HSV at baseline. In all groups, Imlygic was administered based on diameter of the injected tumor:
tumors ≤ 1.5 cm received up to 1 mL of Imlygic,
tumors > 1.5 to ≤ 2.5 cm received up to 2 mL, and
tumors > 2.5 cm received up to 4 mL.
It is notable that the study initially was designed to include all patients (i.e., HSV-seronegative and HSV-seropositive) in 108 pfu/mL exposure groups, but results in the early cohorts identified unacceptable local inflammatory reactions at 107 pfu/mL in HSV-seronegative patients; thus, high-dose groups were limited to HSV-seropositive patients.
D. Imlygic’s Phase 2 Study
Phase 2 testing of Imlygic was conducted in 50 patients with Stage III/IV melanoma.[25] Patients received an initial dose of up to 4 mL of Imlygic (106 pfu/mL) that was followed 3 weeks later with up to 4 mL at 108 pfu/mL administered q2w thereaher for up to 24 treatments. The initial low dose was intended to seroconvert HSV-negative patients to HSV-positive status, thereby avoiding unacceptable reactions in HSV-negative patients. Up to 10 lesions were injected per treatment cycle, using a dosing schema modified from Phase 1:
tumors 0.5 to 1.5 cm in diameter received 0.5 mL of Imlygic,
tumors 1.5 to 2.5 cm received up to 2 mL, and
tumors > 2.5 cm received up to 2 mL.
A maximum of 4 mL of Imlygic could be administered per cycle. Response was assessed by RECIST, starting after six treatment cycles, then every 12 weeks. Overall, 26% of patients achieved an objective response (8 CR, 5 PR, the majority of these having Stage III or M1a disease), and adverse experiences were limited primarily to transient flu-like symptoms.
E. Imlygic’s Phase 3 Trial
Pivotal testing compared IL Imlygic to subcutaneous GM-CSF in 436 patients (randomized 2:1) with unresectable Stage IIIB to IV melanoma.[26] Patients were required to have at least one cutaneous, subcutaneous, or nodal lesion or aggregation of up to 5 lesions with a sum of diameters ≥10 mm that were suitable for injection. As in phase 2, patients received initial injection at 106 pfu/mL followed by subsequent doses at 108 pfu/mL q2w starting 3 weeks aher the initial dose. The dose schema was again modified from prior work, using the following algorithm:
tumors up to 0.5 cm in diameter received up to 0.1 mL of Imlygic,
tumors 0.5 to 1.5 cm received up to 0.5 mL,
tumors 1.5 to 2.5 cm received up to 1.0 mL,
tumors 2.5 to 5 cm received up to 2.0 mL, and
tumors > 5 cm received up to 4 mL.
No minimum size was set for injection, while tumors >10 cm, or patients with tumor burden in excess of 20 cm (sum of longest diameters), required prior approval of the study medical monitor.[27] A maximum of 4 mL per lesion and per treatment cycle was retained from prior work. The primary endpoint was durable response rate (DRR, defined as an objective response lasting continuously ≥6 months), with OS as a key secondary endpoint. DRR in the Imlygic arm was 16.3% vs 2.1% for SQ-GM-CSF (P < 0.001), while median OS was 23.3 months vs 18.9 months (HR = 0.79, P = 0.051). Imlygic was approved based on the results of this study.
F. Imlygic PI
The prescribing information for Imlygic specifies a dose schema identical to the Phase 3 schema.
G. Additional OVs
These studies have led to study of more recently of OVs that leverage predicate work and contemporary development of Imlygic. As with these other examples, dose of OV in pfu is adjusted to maximize oncolytic effect, generally with volume of injectate adapted to facilitate delivery of the desired viral titer.
a. Myb34.5
Gayral et al. tested an investigational oncolytic virus (a targeted HSV-1, Myb34.5) in mouse models of PDAC (human PDAC-derived Mia PACA-2 or Mia PACA-2 Lucia cells implanted in the tail of the pancreas of athymic mice).[28] Tumors were injected once with the OV (106 to 108 pfu, volume not reported) when they reached 150 mm3 (control animals received PBS). Tumor necrosis, hemorrhage, viral replication, and cancer cell apoptosis were observed within two weeks of injection. When the OV was combined with chemotherapy (gemcitabine at 125 mg/kg q3d x 2wk), marked reduction in tumor growth was noted. Neither regimen elicited evidence of significant toxicity.
b. ParvOryx
Hajda et al. conducted a Phase 2 study of an oncolytic H-1 parvovirus (H-1PV, ParvOryx) at 3 escalating doses (109, 5x109, and 1010 pfu total administered dose) in 7 adult patients with confirmed PDAC and at least one hepatic metastasis who had progressed on FOLFIRINOX and were candidates for gemcitabine.[29] Patients received an initial infusion of H-1PV (40ti of total dose given in equal fractions qd x 4) then the remaining dose was administered intralesionally under ultrasound guidance 6-13 days later (administered “as slowly as possible”, injectate volume not specified).[30] Gemcitabine was commenced on day 28 at 1000 mg/m2 BSA, with Nab-paclitaxel (125 mg/m2 BSA) added in the event of disease progression. All dose levels were tolerated, with a median PFS of 2.4 months and a median OS of 5.8 months. While the authors don’t disclose details on injection volume, in predicate non-clinical models, HA-RPC tumors implanted in the pancreatic tail of immunocompetent rats were injected intraoperatively with 100 µL of H-1PV when they reached a size of 150 mm3.[31]
c. IL-12 OV
Barton et al. report on a phase 1 dose escalation study where a replication-competent oncolytic adenovirus modified to express IL-12 (Ad5-yCD/mutTKSR39rep-hIL-12 adenovirus) was injected into metastatic PDAC tumors under ultrasound guidance; 12 subjects received 1011 to 1012 pfu administered to their primary tumor in a volume equivalent to 10ti of tumor mass, with 5fluorcytosine followed by FOLFIRINOX or GEM/NAB starting 21 days after OV injection.[32] The study MTD was not reached, with a median OS of 18.1 months (range 3.5-20.0) observed in the high-dose cohort.
d. HF10 (TaKaRa Bio Inc.)
HF10 is a replication-competent OV derived from HSV-1. Andtbacka et al. reported results from a Phase 2 study of HF10 combined with ipilimumab in patients with Stage IIIB-IV cutaneous melanoma.[33] HF10 was administered at 107 pfu/mL, at up to 5.0 mL, with injection volume adjusted based on size of tumor mass (no further details provided). The authors concluded that the regimen “demonstrated a favorable benefit/risk profile and encouraging antitumor activity.”
H. Additional Non-OV IL Agents
a. SD-101 (Dynavax)
SD-101 is a Toll-like receptor 9 agonist that has been administered via IL injection to several solid tumor types.
Cohen et al. reported results from a Phase 2 study of SD-101 in combination with pembrolizumab in treatment-naïve HNSCC.[34] SD-101 was administered into two dose cohorts as 2 mg injected into 1-4 lesions or 8 mg injected into a single lesion weekly x 4 doses then q3w x 7 doses (28 patients received 2 mg and 23 received 8 mg per injection). One quarter of patients experienced grade ≥3 treatment emergent adverse events (TEAEs), with an ORR of 24% (2 CR and 10 PR).
Similar results were reported by Ribas et al. for the phase 1b portion of a phase 1b/2 study (co-sponsored by Dynavax and Merck as Keynote-184/SYNERGY-001) of SD-101 in combination with pembrolizumab in 22 patients with advanced melanoma; in phase 1b, SD-101 was administered at 1, 2, 4, or 8 mg weekly x 4 doses then q3w x 7 doses. [35] Adverse events appear to have been mostly mild to moderate, with modest clinical outcome (an estimated 12-month PFS of 88% and an ORR of 15%).
Further specifics on treatment parameters were not provided.
b. Tilsotolimod (Idera)
Tilsotolimod (IMO-2125) is an investigational synthetic oligonucleotide which binds to TLR9, improving tumor antigenicity.
Haymaker et al. reported results of a phase 1/2 trial in combination with ipilimumab in 62 patients with anti-PD-1-resistant advanced melanoma.[36] In Phase 1, patients received tilsotolimod at 4 mg (N = 3 patients), 8 mg (9), 16 mg (3), and 32 mg (3), while in phase 2 tilsotolimod was administered to 44 patients at 8 mg; IL injections were administered weekly x 3 weeks then q3w x 3 cycles, then q6w, for a total of nine doses. The total injected dose remained constant, with injection volume adjusted depending on size of injected tumor (no additional details provided). Safety was manageable (48% of patients experienced grade 3 or 4 TEAEs) with a 22% overall response rate in Phase 2.
In an ongoing Phase 1 study in the Netherlands (PANFIRE III), a single IL injection of tilsotolimod (8 mg) is being studied in combination with irreversible electroporation (IRE) and nivolumab in patients with mPDAC; IRE is conducted 1 week after tilsotolimod administration, with nivolumab commenced 2 weeks later.[37] A total of 18 patients are planned for three total arms of the study, with tilsotolimod administered using a perforated needle (ProFusion).
c. Darleukin (L19-IL2, Philogen)
Darleukin is a synthetic immunocytokine comprised of the antibody L19 and the cytokine IL2 designed to accumulate in tissue where angiogenesis occurs and the EDB-FN antigen is expressed.
Weide et al. reported results from a Phase 2 study of darleukin administered to 25 patients with Stage IIIB-IIIC cutaneous melanoma.[38] Darleukin was administered once weekly via IL injection at a dose of 106 IU of IL2 equivalents dissolved in 6 mL of vehicle, with total daily dose divided between all injectable lesions (all lesions present at screening were injected). A CR by modified immune-related response criteria (irRC) was achieved in 6 patients, and 44ti of injected lesions achieved a CR. Toxicity was similar to systemic IL2 (Aldesleukin).
Danielli et al. reported results from a phase 2 study of darleukin administered to 22 patients with Stage IIIC-IVM1a cutaneous melanoma.[39] Darleukin (106 IU IL2 equivalents) and Fibromun (78-312 µg of L19-TNF at investigator’s discretion) were mixed to a final volume of 4.2 mL, and administered once weekly for up to 4 weeks via IL injection, with total daily dose divided between all injectable lesions (all lesions present at screening were injected, along with new lesions that occurred within the regional field during the treatment phase). CR was observed in 1 patient, with PR in an additional 10 patients. Adverse events were mostly mild to moderate.
PV-10
Similar to the algorithms used for ONYX-015 (A.f. above) and Imlygic (C-E above) , PV-10 dosing is based on a space filling model where the ratio of PV-10 volume is matched to the tumor volume (“fill factor”) to deliver a constant dose of rose bengal sodium (RBS) per quantity of tumor (mg RBS / mg tumor).
Non-clinical Fill Factor
In non-clinical testing, Provectus showed that PV-10 yielded maximal benefit in a dose-dependent manner in two sponsored studies of A375 radiation-resistant human melanoma xenographs that documented clinical outcome and overall survival for investigational formulations ranging from 0.1% to 10% RBS (Study A375-PH-10.03, Study A375-e64, and Study 040216.01C), with volume equivalent to approximately 50% of tumor volume. This work established 10% RBS as the standard concentration for PV-10 in all ensuing studies.
Research on PV-10 by the University of Calgary in xenograph models of relapsed and refractory human neuroblastoma substantiated — i.e., reproduced/repeated — that IL injection of PV-10 at approximately 0.4 to 0.8 µL/mm3 estimated tumor volume (i.e., administration of a volume of PV-10 equivalent to 40% to 80% of estimated tumor volume) increased survival in a dose-dependent manner.[40]
Clinical Fill Factor
In initial clinical testing, Thompson et al. reported results from a 20-patient Phase 1 study of PV-10 administered as single IL injections to up to 20 cutaneous melanoma lesions at a dose of 0.5 mL PV-10/cm3 lesion volume (10% RBS; volume equivalent to 50% of tumor volume).[41]
In a follow-up 80-patient study, Thompson et al. reported results from Phase 2 testing of PV-10 administered as repeat IL injections (1-4 injections per lesion administered 4 or more weeks apart); up to 20 cutaneous melanoma lesions were injected at a dose of 0.5 mL PV-10/cm3 lesion volume (10% RBS; volume equivalent to 50% of tumor volume), with a limit of 15 mL (1,500 mg of RBS) at each treatment cycle.[42]
A Phase 1 Study to Assess the Safety and Tolerability of PV-10 in Combination with Gemcitabine/Nab-paclitaxel in Metastatic Pancreatic Adenocarcinoma
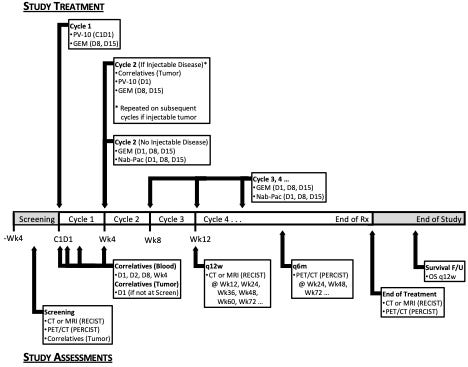
a. Dose escalation/dose expansion flow diagram
PV-10 dosing (i.e., fill factor) will escalate to a “high dose” volume equivalent of 50% of tumor volume.
b. Timeline of study treatment and assessments
Forward-Looking Statements
The information provided in this Provectus Substack Post may include forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, relating to the business of Provectus and its affiliates, which are based on currently available information and current assumptions, expectations, and projections about future events and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Such statements are made in reliance on the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are often, but not always, identified by the use of words such as “aim,” “likely,” “outlook,” “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “would,” “project,” “projection,” “predict,” “potential,” “targeting,” “intend,” “can,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of Provectus’s drug agents and/or their uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this Provectus Substack Post are made as of the date hereof or as of the date specifically specified herein, and the Company undertakes no obligation to update or revise any forward-looking statements, whether because of new information, future events, or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including those described in Item 1A of Provectus’ Annual Report on Form 10-K for the period ended December 31, 2023.
[1] Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015 Sep 1;33(25):2780-8. https://doi.org/10.1200/JCO.2014.58.3377.
[2] Mastrangelo MJ, Sulit HL, Prehn LM, et al. Intralesional BCG in the treatment of metastatic malignant melanoma. Cancer. 1976 Feb;37(2):684-92. https://doi.org/10.1002/1097-0142(197602)37:2<684::aidcncr2820370212>3.0.co;2-y.
[3] Cohen MH, Jessup JM, Felix EL, et al. Intralesional treatment of recurrent metastatic cutaneous malignant melanoma: a randomized prospective study of intralesional Bacillus Calmette-Guerin versus intralesional dinitrochlorobenzene. Cancer. 1978 Jun;41(6):2456-63. https://doi.org/10.1002/10970142(197806)41:6<2456::aid-cncr2820410654>3.0.co;2-b.
[4] Herr HW, Morales A. History of bacillus CalmeWe-Guerin and bladder cancer: an immunotherapy success story. J Urol. 2008 Jan;179(1):53-6. https://doi.org/10.1016/j.juro.2007.08.122.
[5] Gonzalez R, Hutchins L, NemunaiFs J, et al. Phase 2 trial of AllovecFn-7 in advanced metastaFc melanoma. Melanoma Res. 2006 Dec;16(6):521-6. hWps://doi.org/10.1097/01.cmr.0000232299.44902.41.
[6] Bedikian AY, Richards J, Kharkevitch D, et al. A phase 2 study of high-dose AllovecFn-7 in paFents with advanced metastaFc melanoma. Melanoma Res. 2010 Jun;20(3):218-26. hWps://doi.org/10.1097/CMR.0b013e3283390711.
[7] A Phase 3 Pivotal Trial Comparing Allovectin-7® Alone vs Chemotherapy Alone in Patients With Stage 3 or Stage 4 Melanoma. https://www.clinicaltrials.gov/study/NCT00395070.
[8] Agarwala SS. Intralesional therapy for advanced melanoma: promise and limitation. Curr Opin Oncol. 2015 Mar;27(2):151-6. https://doi.org/10.1097/CCO.0000000000000158.
[9] Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000 May;7(10):859-66. https://doi.org/10.1038/sj.gt.3301184.
[10] Rodriguez R, Schuur ER, Lim HY, et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997 Jul 1;57(13):255963.
[11] DeWeese TL, van der Poel H, Li S, Mikhak B, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001 Oct 15;61(20):7464-72.
[12] Markert JM, Medlock MD, Rabkin SD, et al. CondiFonally replicaFng herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000 May;7(10):867-74. https://doi.org/10.1038/sj.gt.3301205.
[13] Friedman GK, Johnston JM, Bag AK, et al. OncolyFc HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N Engl J Med. 2021 Apr 29;384(17):1613-1622. https://doi.org/10.1056/NEJMoa2024947.
[14] Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resecFon for recurrent GBM. Mol Ther. 2009 Jan;17(1):199-207. https://doi.org/10.1038/mt.2008.228.
[15] Markert JM, Razdan SN, Kuo HC, et al. A phase 1 trial of oncolyFc HSV-1, G207, given in combinaFon with radiaFon for recurrent GBM demonstrates safety and radiographic responses. Mol Ther. 2014 May;22(5):1048-55. https://doi.org/10.1038/mt.2014.22.
[16] Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selecFvely in p53-deficient human tumor cells. Science. 1996 Oct 18;274(5286):373-6. https://doi.org/10.1126/science.274.5286.373.
[17] Heise C, Sampson-Johannes A, Williams A, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and anti-tumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997 Jun;3(6):639-45. https://doi.org/10.1038/nm0697-639.
[18] This work by Von Hoff and colleagues used tumor models and treatment schemes equivalent to those used in early preclinical contract work by Von Hoff on PV-10.
[19] Ganly I, Kirn D, Eckhardt G, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000 Mar;6(3):798-806.
[20] Mulvihill S, Warren R, Venook A, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001 Feb;8(4):308-15. https://doi.org./10.1038/sj.gt.3301398.
[21] Hecht JR, Bedford R, Abbruzzese JL, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003 Feb;9(2):55561.
[22] NemunaiFs J, Khuri F, Ganly I, et al. Phase II trial of intratumoral administration of ONYX-015, a replicationselective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001 Jan 15;19(2):289-98. https://doi.org/10.1200/JCO.2001.19.2.289.
[23] Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003 Feb;10(4):292-303. https://doi.org/10.1038/sj.gt.3301885.
[24] Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006 Nov 15;12(22):6737-47. https://doi.org/10.1158/1078-0432.CCR-06-0759.
[25] Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009 Dec 1;27(34):5763-71. https://doi.org/10.1200/JCO.2009.24.3675.
[26] Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in PaFents With Advanced Melanoma. J Clin Oncol. 2015 Sep 1;33(25):2780-8. https://doi.org/10.1200/JCO.2014.58.3377.
[27] BioVex clinical study protocol 005/05, Andtbacka et al. JCO 2015 ibid, supplemental materials.
[28] Gayral M, Lulka H, Hanoun N, et al. Targeted oncolytic herpes simplex virus type 1 eradicates experimental pancreatic tumors. Hum Gene Ther. 2015 Feb;26(2):104-13. https://doi.org/10.1089/hum.2014.072.
[29] Hajda J, Leuchs B, Angelova AL, et al. Phase 2 Trial of Oncolytic H-1 Parvovirus Therapy Shows Safety and Signs of Immune System Activation in Patients With Metastatic Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2021 Oct 15;27(20):5546-5556. https://doi.org/10.1158/1078-0432.CCR-21-1020.
[30] Hajda J, Lehmann M, Krebs O, et al. A non-controlled, single arm, open label, phase II study of intravenous and intratumoral administration of ParvOryx in patients with metastatic, inoperable pancreatic cancer: ParvOryx02 protocol. BMC Cancer. 2017 Aug 29;17(1):576. https://doi.org/10.1186/s12885-017-3604-y.
[31] Angelova AL, Aprahamian M, Grekova SP, et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res. 2009 Jan 15;15(2):511-9. https://doi.org/10.1158/1078-0432.CCR-08-1088.
[32] Barton KN, Siddiqui F, Pompa R, et al. Phase I trial of oncolytic adenovirus-mediated cytotoxic and interleukin-12 gene therapy for the treatment of metastatic pancreatic cancer. Mol Ther Oncolytics. 2020 Dec 3;20:94-104. https://doi.org/10.1016/j.omto.2020.11.006.
[33] Andtbacka RHI, Ross MI, Agarwala SS, et al. Final results of a phase II multicenter trial of HF10, a replicationcompetent HSV-1 oncolytic virus, and ipilimumab combination treatment in patients with stage IIIB-IV unresectable or metastatic melanoma. ASCO 2017 Annual Meeting. J Clin Oncol 35, 2017 (suppl; abstr 9510). https://doi.org/10.1200/JCO.2017.35.15_suppl.9510.
[34] Cohen EEW, Nabell L, Wong DJ, et al. Intralesional SD-101 in Combination with Pembrolizumab in AnF-PD-1 Treatment-Naïve Head and Neck Squamous Cell Carcinoma: Results from a Multicenter, Phase II Trial. Clin Cancer Res. 2022 Mar 15;28(6):1157-1166. https://doi.org/10.1158/1078-0432.CCR-21-1411.
[35] Ribas A, Medina T, Kummar S, et al. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discov. 2018 Oct;8(10):1250-1257. https://doi.org/10.1158/2159-8290.CD18-0280.
[36] Haymaker C, Johnson DH, Murthy R, et al. Tilsotolimod with Ipilimumab Drives Tumor Responses in AnF-PD-1 Refractory Melanoma. Cancer Discov. 2021 Aug;11(8):1996-2013. https://doi.org/10.1158/2159-8290.CD-20-1546.
[37] Geboers B, Timmer FEF, Ruarus AH, et al. Irreversible Electroporation and Nivolumab Combined with
Intratumoral Administration of a Toll-Like Receptor Ligand, as a Means of In Vivo Vaccination for Metastatic Pancreatic Ductal Adenocarcinoma (PANFIRE-III). A Phase-I Study Protocol. Cancers (Basel). 2021 Aug 2;13(15):3902. https://doi.org/10.3390/cancers13153902.
[38] Weide B, Eigentler TK, Pflugfelder A, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014 Jul;2(7):66878. https://doi.org/10.1158/2326-6066.CIR-13-0206.
[39] Danielli R, Patuzzo R, Di Giacomo AM, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015 Aug;64(8):999-1009. https://doi.org/10.1007/s00262-015-1704-6.
[40] Swis L, Zhang C, TrippeW T, Narendran A. Potent in vitro and xenograft antitumor activity of a novel agent, PV-10, against relapsed and refractory neuroblastoma. Onco Targets Ther. 2019 Feb 18;12:1293-1307. https://doi.org/10.2147/OTT.S191478.
[41] Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008 Dec;18(6):405-11. https://doi.org/10.1097/CMR.0b013e32831328c7.
[42] Thompson JF, Agarwala SS, Smithers BM, et al. Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma. Ann Surg Oncol. 2015 Jul;22(7):2135-42. https://doi.org/10.1245/s10434-014-4169-5.