Provectus Provides Updated PV-10 Overall Survival Data on Hepatic Metastatic Uveal Melanoma
Provectus last provided an update on PV-10 data for its uveal melanoma (UM) metastatic to the liver (mUM) clinical trial in Provectus’s November 2023 Substack post titled “Provectus Provides Updated PV-10 Data on Metastatic Uveal Melanoma” that used a data cutoff of August.
Stage M1a
Of the study’s 25 patients, 17 (68%) were Stage M1a. Of these 17 patients:
Seven (41%) received the combination therapy of PV-10 + anti-CTLA-4 (ipilimumab [“ipi”]) + anti-PD-1 (nivolumab [“nivo”]) (“+IN” or “+COMB”), of whom
Ten (59%) received PV-10 only or PV-10 + anti-PD-1 (“-IN” or “-COMB”), and
Four (24%) achieved complete metabolic response (CMR) and remain alive as of this reporting.
Of the seven patients who received PV-10 + ipi + nivo (PV-10 + IN), three (43%) achieved CMR.
Of the ten patients who did not receive IN, one (10%) achieved CMR.
Each of the four mUM CMRs received PV-10 injections to multiple uveal melanoma liver metastases: a median of 2 tumors injected with PV-10 and a median of 1.5 injection cycles of drug, while patients in Provectus’s mUM study as a whole had a median of 2 tumors injected and a median of 2 injection cycles. This contrasts with prior Provectus liver metastases clinical development programs in hepatocellular carcinoma (HCC), colorectal cancer (mCRC), and neuroendocrine tumors (mNET), where patients had a median of 1 PV-10-injected tumor and a median of 1 PV-10 injection cycle.
Stage M1a Overall Survival
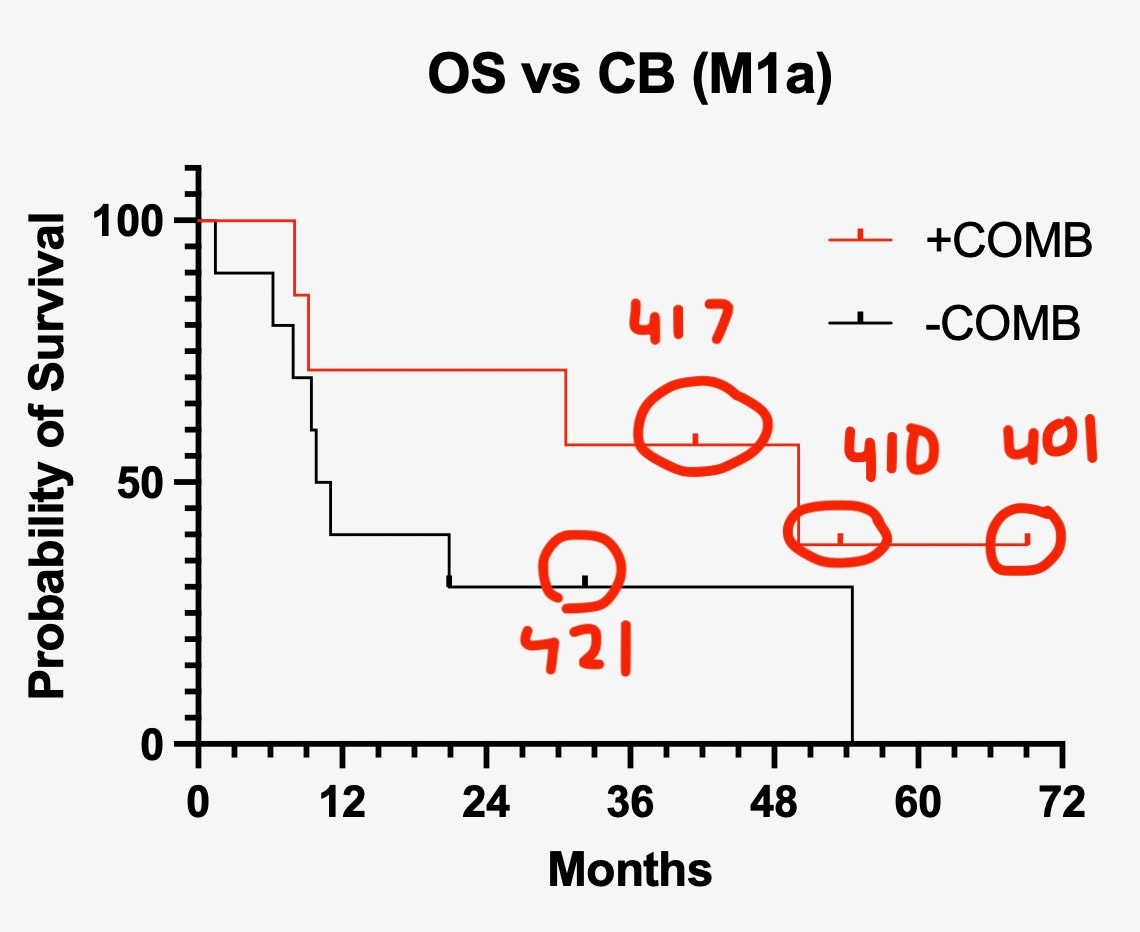
Estimated median overall survival (OS) (mOS) of PV-10 + IN (+COMB) is holding at 50 months, unless subject 0417 (who is currently at an OS duration of 43 months) expires before reaching month 50 (September 2024). Since four of seven subjects in this category have expired, mOS will be confirmed at 50 months if 0417 surpasses the milestone date, or will be Patient 0417’s OS duration if they expire before then. If 0417 surpasses month 50, the tail of the survival curve will be 38% OS for >4 years.
Comparing survival curves of +COMB (i.e., +IN; patients who received PV-10 + ipi + nivo) and -COMB (i.e., -IN; patients who did not), see the graph below.
Because there are not a lot of +IN; +COMB patients (N=7) and relatively few -IN; -COMB patients (N=10), the difference in the survival curves is not statistically significant (p=0.2). The hazard ratio (HR), which is a measure of difference in the shape of the curves, however, is HR=0.47. From Wikipedia’s Hazard Ratio entry: “In its simplest form, the hazard ratio can be interpreted as the chance of an event occurring in the treatment arm divided by the chance of the event occurring in the control arm, or vice versa, of a study.” HR=0.47 means patients in the PV-10+IN; +IN; +COMB arm were approximately half as likely to die as those in the PV-10-IN; -IN; -COMB arm.
Takeaways
During Provectus’s First Quarter 2024 conference call, the Company said, “We believe enough PV-10 injections enough times to enough lesions for long enough can lead to durable systemic responses and long-term outcomes for patients. A little PV-10 can go a long way, but enough PV-10 might cure you.”
How do these mUM data, and these mUM CMRs, potentially shape this comment?
At its most basic, comparing the survival curves of PV-10 + IN versus PV-10 - IN potentially yields an “activity signal” PV-10 + IN.
Again, the curves in Figure 2 are two PV-10 treatment arms.
What would be the estimated or target HR for PV-10 + IN versus IN in Stage M1a mUM patients with hepatic or hepatic/extra-hepatic disease?
For IN, see Pelster et al. 2021.
Forward-Looking Statements
The information provided in this Provectus Substack Post may include forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, relating to the business of Provectus and its affiliates, which are based on currently available information and current assumptions, expectations, and projections about future events and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Such statements are made in reliance on the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are often, but not always, identified by the use of words such as “aim,” “likely,” “outlook,” “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “would,” “project,” “projection,” “predict,” “potential,” “targeting,” “intend,” “can,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of Provectus’s drug agents and/or their uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this Provectus Substack Post are made as of the date hereof or as of the date specifically specified herein, and the Company undertakes no obligation to update or revise any forward-looking statements, whether because of new information, future events, or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including those described in Item 1A of Provectus’ Annual Report on Form 10-K for the period ended December 31, 2022 and the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2023.