Stage IV Melanoma: “…the company that saved my life”
A patient testimonial about his investigational cancer immunotherapy PV-10 experience
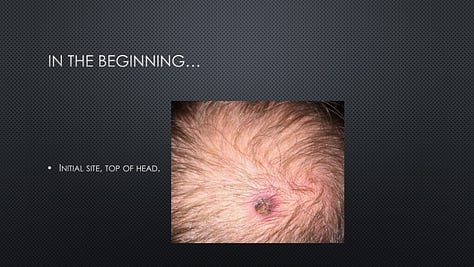
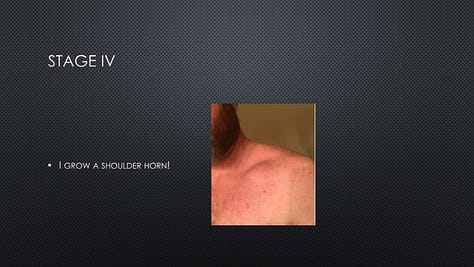
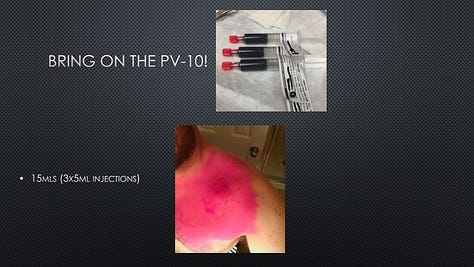
In June 2019, a patient (“Tex”) in Provectus’s Phase 1b clinical trial of cancer immunotherapy PV-10 and immune checkpoint inhibitor Keytruda® (pembrolizumab) for checkpoint-naïve Stage IV melanoma (i.e., the main cohort of multi-cohort NCT02557321) reached out to the Company to ask if he could speak at Provectus’s 2019 annual stockholder meeting in Knoxville, Tennessee.
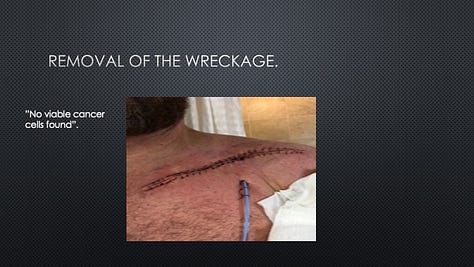
In 2020, Provectus presented 2-year landmark survival, response, safety, and other data from Tex’s clinical trial at the European Society for Medical Oncology (ESMO) Virtual Congress, including 62% overall survival (OS) (55% for historical pembrolizumab alone) and median OS (mOS) not reached (mOS also not reached for historical pembrolizumab alone). A link to a copy of the ESMO poster presentation may be found here. The study also achieved an overall response rate (ORR; by RECIST) of 67%, a 10% complete response (CR), and an estimated progression-free survival (PFS) of 11.7 months.
Tex, who by 2018 had achieved CR with no evidence of disease (NED), wanted to share his story with shareholders and his praise for the impact that he felt PV-10 had had on the course of his disease, his treatment outcome, and ultimately his life. Tex provided us with the presentation that he wanted to give.




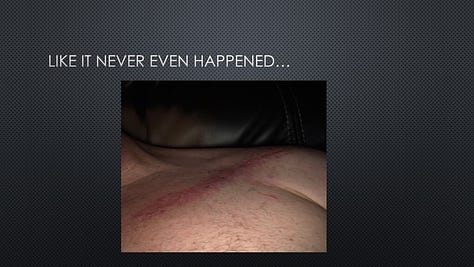
At the time, there was an internal debate at Provectus about permitting Tex to speak at the shareholder meeting. Some very much welcomed his patient testimonial. Others were concerned about the potential consequences of Tex’s presentation, including that he was an active clinical trial participant, the possible appearance of inappropriate inducement (because his proposed interaction with Provectus [i.e., a presentation during the stockholder meeting] was different from that of other study participants and had not been reviewed by his treating hospital’s institutional review board [IRB]), possible implications with regard to objectivity (both for him and the Company), and that the distribution of his presentation (i.e., Provectus would have had to include the presentation in its Form 8-K that we typically make for stockholder meeting presentation slides) could be construed as an unapproved promotion of an unapproved investigational drug and/or unapproved efficacy claims.
While we believe that intent matters (i.e., Tex simply wanted to share his story with Provectus shareholders), we were approximately two years into the rescue and turnaround of the Company, and arrived at a consensus decision to not provide a formal venue for Tex to speak and present. Tex, however, did introduce himself to and spoke with shareholders after the 2019 meeting concluded. The clinical trial, and its three patient cohorts, have since closed to enrollment.
It should be noted that Tex has consistently shared with us over time that he owes his successful treatment and life to PV-10.
Tex reached out to Provectus in December 2023 to note that he still was a CR with NED, about six-and-a-half years since he began the combination therapy treatment of PV-10 and pembrolizumab at MD Anderson Cancer Center in Houston, Texas. He is now racing in the 2024 Ferrari Racing Challenge:
Round 1: Circuit of the Americas: April 24 – 28
Round 2: Laguna Seca: May 15 – 19
Round 4: Watkins Glen International: July 10 – 14
Round 5: Sonoma Raceway: August 21 - 25
Round 6: Indianapolis Motor Speedway: September 11 - 15
Round 7 - Finali Mondiali: Fall 2024
The finale (the Finali Mondiali) will be held in Italy in the fall for the top drivers of the season.
Tex asked us if he could include the Provectus logo on his race car for the upcoming season (at no charge to the Company). He simply was excited about his racing opportunity, and wanted to bring more exposure to Provectus. Logo placement is on both rear fenders behind the wheels (the spoiler was reserved by Ferrari Of San Antonio, the dealership for whom he races) and on his fire suit.
He provided us with an update in early-April. He will be competing in the Coppa Shell AM class of the Ferrari Challenge series. Tex was progressing through the Ferrari driving program (Corso Pilota) and knew after his second course that he wanted to race. Tex happened to meet the director of Ferrari North America motorsports at this course, who offered him not just any Ferrari race car but the very last Ferrari 488 Challenge Evo race car ever built. It was on the assembly line at the factory in Maranello when the director made the offer. Five months later, Tex’s ride arrived fresh off the boat. That car will remain with Tex even after they transition to the newer Ferrari 296 Challenge car in 2025.




Tex’s patient testimonial: “I’m proud to represent the company that saved my life and bring any attention I can to you…”
Provectus is grateful for patients like Tex who want to share their stories about their involvement in the Company’s clinical trials. Tex gave Provectus his permission to write this Substack post, and he reviewed it before it was published.
The Company is obviously not promoting an unapproved investigational drug or an unapproved efficacy claim.
Go Tex, and thank you!
Forward-Looking Statements
The information provided in this Provectus Substack Post may include forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, relating to the business of Provectus and its affiliates, which are based on currently available information and current assumptions, expectations, and projections about future events and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Such statements are made in reliance on the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are often, but not always, identified by the use of words such as “aim,” “likely,” “outlook,” “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “would,” “project,” “projection,” “predict,” “potential,” “targeting,” “intend,” “can,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of Provectus’s drug agents and/or their uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in this Provectus Substack Post are made as of the date hereof or as of the date specifically specified herein, and the Company undertakes no obligation to update or revise any forward-looking statements, whether because of new information, future events, or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including those described in Item 1A of Provectus’ Annual Report on Form 10-K for the period ended December 31, 2023.



