The Impact of COVID-19 on Provectus’s Research & Development, Halogenated Xanthene Medical Science Platform, and Rose Bengal Sodium-based Investigational Drug & Drug Formulation Pipeline
Originally tweeted February 17 to March 8, 2023 + Some new commentary
This Substack post discusses the impact of the coronavirus disease 2019 (COVID-19) global pandemic on Provectus’s R&D, drug discovery, and clinical development activities. We’ll endeavor to describe several aspects of the journey of our lead molecule Rose Bengal Sodium (RBS) to potentially becoming a multi-disease, broad-spectrum therapeutic in this post.
COVID-19 has been a deeply painful experience, to say the least, for the U.S. and the world. It also marked a turning point in the advancement and expansion of Provectus’s Halogenated Xanthene (HX) medical science platform, as well as the Company’s RBS and the investigational drugs and drug formulations derived from it.
In early-March 2020, Provectus’s leadership team discussed what, if any, role our HX medical science platform and RBS could play as a potential therapeutic and/or prophylactic against COVID-19. As a reference, here is a sample timeline of this period.
Provectus believed that RBS might have the potential to be specifically a COVID-19 inhibitor and more broadly a broad-spectrum viral inhibitor. We knew that rose bengal had been shown in the somewhat distant past to demonstrate antiviral in vitro activity, albeit under light activation: herpes simplex virus type 1 (HSV-1), vesicular stomatitis virus (VSV), influenza virus, murine respirovirus (formerly Sendai virus; aka murine parainfluenza virus type 1), etc. We tried to connect this in vitro rose bengal work in virology to Provectus’s extensive clinical and preclinical work in oncology and then-emerging in vivo work in hematology.
Provectus tells this story, in part, via then-new intellectual property that we created: “Novel Uses of Halogenated Xanthenes in Oncology and Virology,” US-20210299083-A1; provisional filed 3/26/20; application filed 3/25/21; published 9/30/21. We contend that oncology and virology are related fields of medical science that intersect at the innate and adaptive immune systems of both animals and humans. While disease etiology and manifestations are generally distinct, this intersection establishes a common basis for the application of discoveries in one field to the other.
Just prior to 2019 year-end, we learned from our research collaborators at the Cumming School of Medicine at the University of Calgary (Aru Narendran, MD, PhD) that, using acute monocytic leukemia (AML) (i.e., hematology-related) and other oncology-related preclinical models, HXs such as RBS may induce acute STING (stimulator of interferon genes) dimerization. This STING work was later presented at the American Association for Cancer Research (AACR) 2020 Virtual Annual Meeting II in June 2020: “Association of Heat Shock Proteins as Chaperone for STING: A potential link in a key immune activation mechanism revealed by a novel anticancer agent PV-10.” STING is an important regulator of innate immunity, and STING dimerization is critical to innate immune system signaling. Acute STING activation via dimerization is required for activity and protective function in both oncology and virology.
Immune function increases rapidly during early childhood and remains consistent across adulthood until the onset of advanced age. Increased incidences of cancers and, maybe mortality from COVID-19 with age, which we thought at the time could be attributable to changes in STING expression and activation with age (i.e., decreased immune system facility may be an important factor in disease severity).
Dr. Narendran’s University of Calgary team and Provectus surmised at least three COVID-19 drug targets:
STING,
the catalytic pocket of COVID-19’s main protease Mpro, and
COVID-19’s characteristic surface spike (S) glycoprotein.
Provectus’s annual stockholder meeting (ASM) presentations from 2018 and 2019 should make it clear that COVID-19 provided a game-changing opportunity starting in 2020 for the Company’s new leadership team to explore, more deeply understand, and better communicate the true expanse of our HX medical science platform and RBS’ true therapeutic potential. Some historical context is necessary: A group of stockholders (PRH), Provectus’ eventual new leadership team, entered into a Definitive Financing with the Company in 2017.
Provectus’s 2018 ASM presentation was an inventory of the known science and clinical and preclinical data that we had to work with at the time. The Company’s 2019 ASM presentation described the beginning of our journey to truly better understand the multi-faceted traits of our lead HX molecule, RBS (see slide no. 17), and assess the known strengths and weaknesses of checkpoint inhibitors for the treatment of cancer.
As PRH finalized the Definitive Financing transaction with Provectus, we green-lit two key projects and sponsored research agreements (SRAs):
Jim Krueger, MD, PhD at The Rockefeller University, and
Dr. Narendran at the University of Calgary.
The SRA with Dr. Krueger and The Rockefeller University in New York, NY was for some initial clinical mechanism work on PH-10, Provectus’s clinical-stage immuno-dermatology agent and psoriasis. The SRA with Dr. Narendran and the University of Calgary in Alberta, Canada was for initial preclinical research on PV-10, Provectus’s clinical-stage cancer immunotherapy agent, and pediatric cancer cell lines.
Because it spanned a wide range of interleukins (i.e., interleukin numbers), Dr. Krueger’s clinical work was consequential to us beginning to potentially bracket RBS’s behavior. Generally speaking, at high concentrations RBS may function as a stimulatory agent, while at low concentrations RBS may function as an anti-inflammatory agent. Over time (i.e., since COVID-19), we realized that this crudish mechanistic description was perhaps too simplistic.
It is noteworthy, for historical context only, that some post-PRH-retained Provectus staff wanted to discard PH-10, and had previously advocated for the shutdown of its further development. What a shame that would have been to the new leadership’s willingness to embrace a vastly more open mind to the medical potentialities and possibilities of RBS, and what is allowed us to dream of and conjecture.
Dr. Narendran’s preclinical research on PV-10 in pediatric solid tumors (e.g., relapsed pediatric neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, osteosarcoma, etc.) was critically important (e.g., in vivo proof-of-concept for refractory and relapsed pediatric solid tumor cancers; replicability of Provectus’s lysosomal targeting mechanism proposition for RBS; reproducibility of PV-10’s combination therapy characteristic in chemotherapy and radiotherapy, etc.).
In vivo proof-of-concept for intratumoral treatment with PV-10 of refractory and relapsed pediatric solid tumor cancers: Swift et al. (2019), “Potent in vitro and xenograft antitumor activity of a novel agent, PV-10, against relapsed and refractory neuroblastoma,”
The replicability of Provectus’s lysosomal targeting in Swift et al.: Wachter et al. (2002), “Imaging Photosensitizer Distribution and Pharmacology using Multiphoton Microscopy,”
The replicability of PV-10’s combination therapy characteristic in chemotherapy from Swift et al.: Dees et al., SITC 2012, and
Finally, the replicability of PV-10’s combination therapy characteristic: radiotherapy: Foote et al. (2017), “Results of a phase II, open-label, non-comparative study of intralesional PV-10 followed by radiotherapy for the treatment of in-transit or metastatic melanoma.”
But, Dr. Narendran became even more consequential to Provectus, which had long since dispensed with wet lab and murine model work for nearly a decade before COVID-19 struck. His lab (the Narendran Lab) essentially became and remains our lab for a range of work that followed and is ongoing:
adult and pediatric oncology,
hematology,
infectious disease (i.e., virology: COVID-19),
vaccines (i.e., RBS as an immune adjuvant),
mechanism of action,
and more (e.g., chaperone-mediated autophagy).
Many healthcare companies (e.g., supplies, devices, pharmaceuticals, etc.) and their teams jumped into the battle against COVID-19. See, for example, this article from that time (2020).
In early-March 2020, Provectus did too. We called Dr. Narendran to ask for his lab team’s and his assistance to evaluate Provectus’s RBS’s potential activity against COVID-19. His team was already on it: in silico study (i.e., computer modeling).



The Narendran Lab calculated initial negative binding energies of -2.5 kcal/mol for the N3 inhibitor, -3.4 kcal/mol for the reference molecule, and -14.3 kcal/mol for PV-10 (otherwise known as Vina scores from AutoDock Vina opensource molecular modeling simulation software). The higher the negative binding energy (i.e., the more negative), the more stable the binding of the molecule to the target site (i.e., the more “activity”).
There are pros, cons, caveats, etc. with in silico work, as there is with in vitro and in vivo studies. See here, for example. Nevertheless, it is important to keep in mind that in silico, in vitro, and in vivo study are perfunctory, customary, and yet very important steps in the drug discovery process. For Provectus, from a therapeutic perspective, with clinical trial and expanded access data in oncology, clinical trial data in dermatology, clinical pilot study data in ophthalmology at Bascom Palmer Eye Institute at the University of Miami, and clinical pilot study data in podiatry at Western Sydney University (Australia), COVID-19 and then later R&D work in different disease areas simply represented starting at the “activity” finish line and working backwards using modern drug discovery techniques, like computer modeling, to backfill PRH’s understanding of small molecule HX RBS.
To be clear, The Narendran Lab’s in silico (i.e., computer modeling) work was the first such study by Provectus, ever…
As we noted earlier in this post, replicability of Provectus’s medical science is very important, and very important to us, because it potentially validates our contention of RBS as real medical science.
During the early months of COVID-19, researchers from Oak Ridge National Laboratory (ORNL), where Provectus’s original founders came from, used ORNL’s supercomputer Summit, via computer modeling (i.e., in silico study), to identify drug candidates that showed promise for treating the virus.
ORNL, February 2020: Smith et al. “Repurposing Therapeutics for COVID-19: Supercomputer-Based Docking to the SARS-CoV-2 Viral Spike Protein and Viral Spike Protein-Human ACE2 Interface,” and
ORNL, March 2020: CNN.
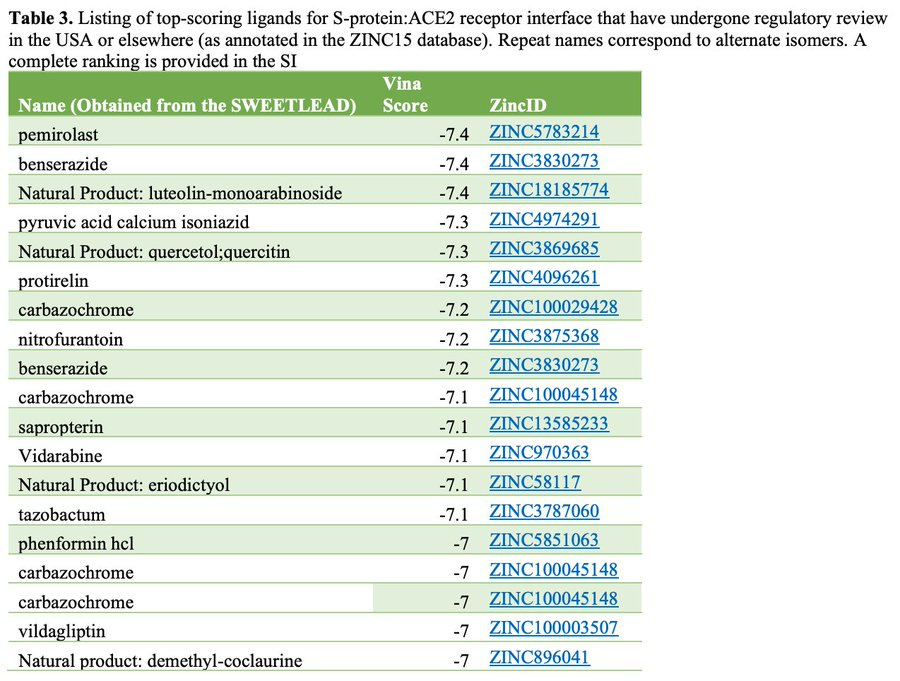
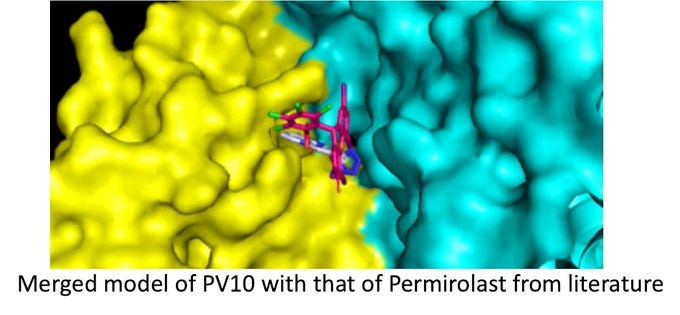
Smith et al. tabulated Vina scores of molecules in the SWEETLEAD database, noting the compound permirolast as having the most negative binding energy (i.e., highest [negative] Vina score). Despite being in the SWEETLEAD database (sort of), Provectus’s RBS did not register in the Smiths’ analyses of Summit’s number-crunching.
Provectus asked Dr. Narendran if his lab team could continue their in silico work with pemirolast from Smith et al.’s and ORNL’s computer modeling work. Dr. Narendran’s team returned an initial perspective of RBS (PV-10) showing a preliminary calculation of negative binding energy equal to or better than pemirolast: -12.5 kcal/mol and -7.2 kcal/mol for Provectus’s PV-10 (again, using the virus target in the Smith et al. paper: the CoV2 Viral Spike Protein and Human ACE2 interface).


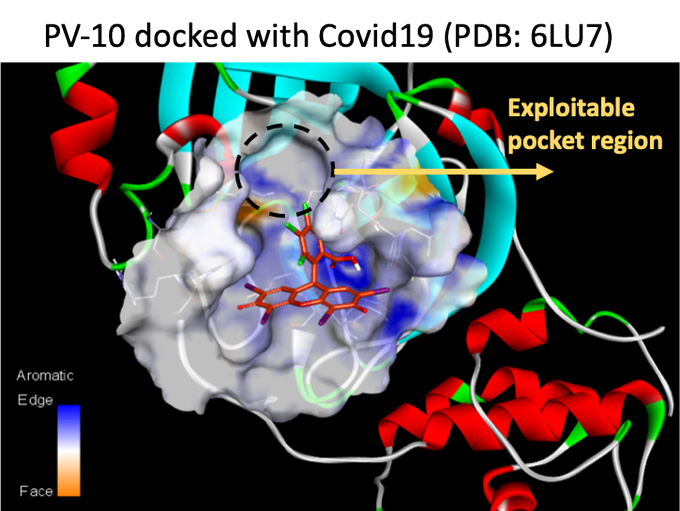
With these data (and knowledge) in hand, Provectus approached ORNL to ask about RBS’s potential in silico activity. ORNL’s subsequent in silico work and calculations indicated that our HX molecule had weak binding affinity towards their then-primary targets. After we spent time with them, ORNL realized that the molecular pose of RBS in the SWEETLEAD database (this version of the molecule is also in the ZINC database) was, um, different (i.e., wrong)…
We suppose that this is what happens when the rest of the world is still using a 19th-century molecule, and Provectus’s RBS is a 21st-century, proprietarily-synthesized, small molecule immuno-catalyst. Put another way, a lot of data, information, knowledge, and eventually wisdom about rose bengal and RBS has been lost to history over the last 140 or more years.
Luckily, even with the somewhat incorrect molecular pose. ORNL noted that their docking calculations suggested stronger binding affinities for RBS at a presumed inhibitor binding site in the MPro protease. Critically, this replicated Dr. Narendran’s team’s initial in silico work. ORNL subsequently redid their in silico work, which conformed with Dr. Narendran’s team’s work on Provectus’s RBS. ORNL then engaged in a blind-docking exercise using RBS against seven more COVID-19 targets (i.e., endoribonuclease, NSP3, two different N-protein fragments, NSP10, NSP16, and NSP9). Provectus’s lead HX molecule appeared to be quite promiscuous, with “good” scores for multiple targets.
What are our points, themes, narratives, etc. of this University of Calgary, Alberta, Canada-ORNL, Tennessee, USA cross-border COVID-19 story?
first and foremost, replicability: obtaining consistent results from different studies (in this case, in silico or computer modeling work) aimed at answering the same scientific question of whether or not there is activity from (i.e., binding of) RBS,
selective promiscuity,
multiple targets,
multiple mechanisms,
combination therapy, and
more…
These themes in virology and infectious disease connect across Provectus’s drug discovery and clinical development work in [at least] oncology, dermatology, and hematology to date. For promiscuousness, see Bojadzic et al. “Toward Small-Molecule Inhibition of Protein–Protein Interactions: General Aspects and Recent Progress in Targeting Costimulatory and Coinhibitory (Immune Checkpoint) Interactions” (page 9).
In 2021, following the COVID-19 computer modeling experiences with the University of Calgary and ORNL, Provectus approached a pharmaceutical services firm to undertake more in silico work.
Computer modeling in drug discovery is, like in vitro work (e.g., high-throughput screening) and in vivo work, a funneling or winnowing exercise. See the images below. Lots of molecules, compounds, ligands, etc. A target (of a disease). What molecule hits the target?
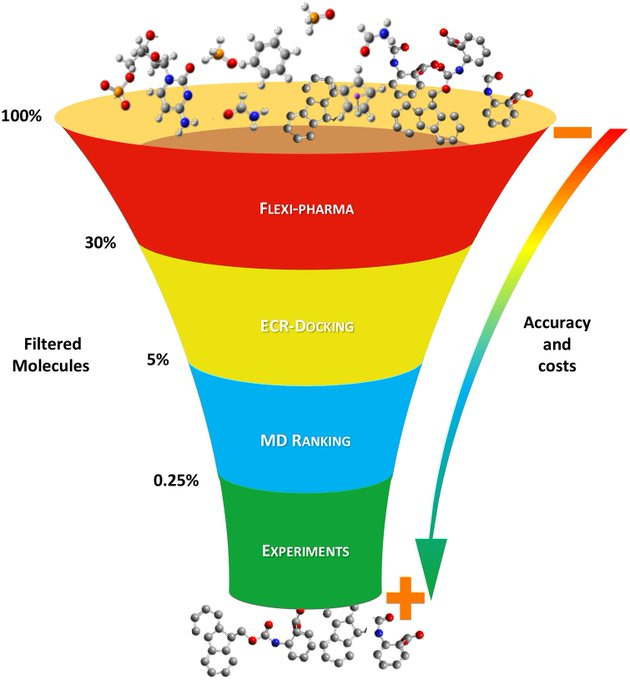

We sought to turn in silico study on its head: One molecule (RBS), lots of targets. One molecule-and-one target is a function of the known knowns (i.e., a known target). A known unknown would be an unvalidated target of a disease (i.e., a new mechanism not yet shown to have therapeutic relevance). And then there would be the unknown unknowns. We initially got some funny looks from the computer modelers when we posed the in silico question of inverting the drug discovery funnel in order to determine what target(s) [of a disease and across diseases] could RBS hit. One target of RBS in this 2021 work, without our prompting, turned out to be STING. There were a handful of other intriguing potential in silico targets.
Dr. Narendran’s lab from the Cumming School of Medicine at the University of Calgary showed, via in vitro work, that PV-10 treatment led to STING dimerization and the release of interferon gamma (IFNγ). This work was presented at AACR 2020. Dr. Narendran’s lab described a previously unidentified mechanism of STING activation by Provectus’s PV-10, which may facilitate sustained immune activation and therapeutic anti-tumor activity.
We understand the knowledge that can come from preclinical research, like in silico, in vitro, and in vivo studies. We also understand the limitations of this aspect of drug discovery, but also the importance of characterizing RBS in customary biopharmaceutical industry terms and settings. Since 2020 and COVID-19, one prong of our business strategy has been to ask the simple question: Does RBS target disease [fill-in-the-blank]?
We believe and think that it does.
SUBSTACK POST END: The Impact of Covid on Provectus’s Research & Development, Halogenated Xanthene Medical Science Platform, and Rose Bengal Sodium-based Investigational Drug & Drug Formulation Pipeline.
Forward-Looking Statements
The information in Provectus’ Substack post may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in Provectus’ Substack are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, and
Provectus’ Quarterly Report on Form 10-Q for the period ended September 30, 2022.