Provectus Announces Presentations at Upcoming Scientific and Medical Conferences
Society for Immunotherapy of Cancer & Society for Melanoma Research
November 2023’s SITC and SMR conferences
As we noted in Provectus’s press releases (PRs) on August 1st and August 7th, three abstracts about investigational cancer immunotherapy PV-10 (rose bengal sodium [RBS]) were accepted to two upcoming 2023 scientific and medical conferences:
Society for Immunotherapy of Cancer (SITC) annual meeting (November 1-5), and
Society for Melanoma Research (SMR) congress (November 6-9).
August 1st PR: PV-10 for uveal melanoma and cutaneous melanoma
Data from ongoing clinical trials of PV-10 for the combination therapy treatments of uveal melanoma (UM) metastatic to the liver (mUM) (NCT00986661) and Stage III-IV cutaneous melanoma (NCT02557321) will be presented on two poster presentations at the SMR annual meeting in Philadelphia, PA.
The accepted SMR abstracts are:
“Percutaneous autolytic immunotherapy for metastatic uveal melanoma in patients with hepatic metastases,” and
“Combination PV-10 autolytic immunotherapy and immune checkpoint blockade in an expanded cohort of checkpoint-naïve cutaneous melanoma patients.”
August 7th PR: PV-10 as an immune vaccine adjuvant
Preclinical data from ongoing research on PV-10 as an immune vaccine adjuvant will be presented on a poster presentation at SITC 2023 in San Diego, CA. This research has been led by Aru Narendran, MD, PhD and his team of researchers at the Cumming School of Medicine of the University of Calgary in Alberta, Canada.
The accepted SITC abstract is:
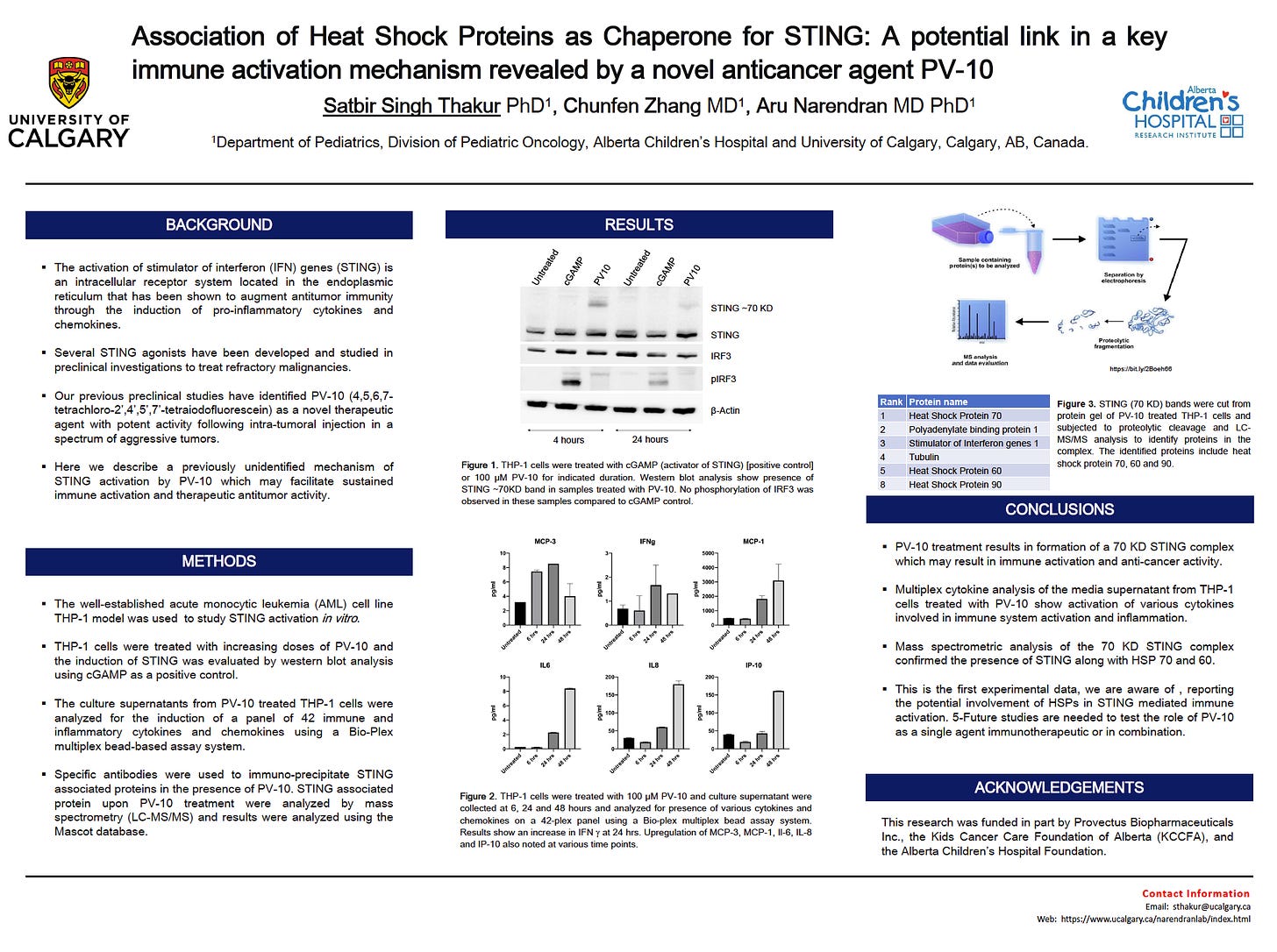
“The iodinated fluorescein derivative PV-10 enhances the antiviral activity of CD8+ T-Cells by inducing STING dimerization: Implications for enhanced vaccine applications.”
The SMR and SITC cancer conference presentations add to Provectus’s current total of 32 presentations (27 posters, 5 orals) from 2017 to 2023 (~5 per year), which compare to 23 prior presentations (all posters, no orals) from 2006 to 2017 (~2 per year).
We prioritized a strategy and the effort of presenting more data more regularly from Provectus’s Part One of PV-10’s clinical development program (CDP) (see Substack post Provectus's 2023 Annual Stockholder Meeting), as well as associated and expanded preclinical study of different therapeutic medical applications of different formulations of RBS. We did this (i.e., present more data more regularly) while identifying and assessing historical process and data gaps from Part One of PV-10’s CDP:
Optimized PV-10 pharmacokinetics (PK),
Well understood, clearly defined, target patient populations,
Study endpoints (EPs) unequivocally supportive of clearly defined trajectories along potential regulatory pathways,
Well-established, well-reasoned, functional EP measurement,
Comprehensive immune correlative assessment undeniably demonstrating immuno-therapeutic outcomes largely due to PV-10 treatment, and
Compelling clinical-business rationales sufficiently addressing standard of care (SOC), potential regulatory pathways, and/or commercial development.
More data are better; longer survival is best
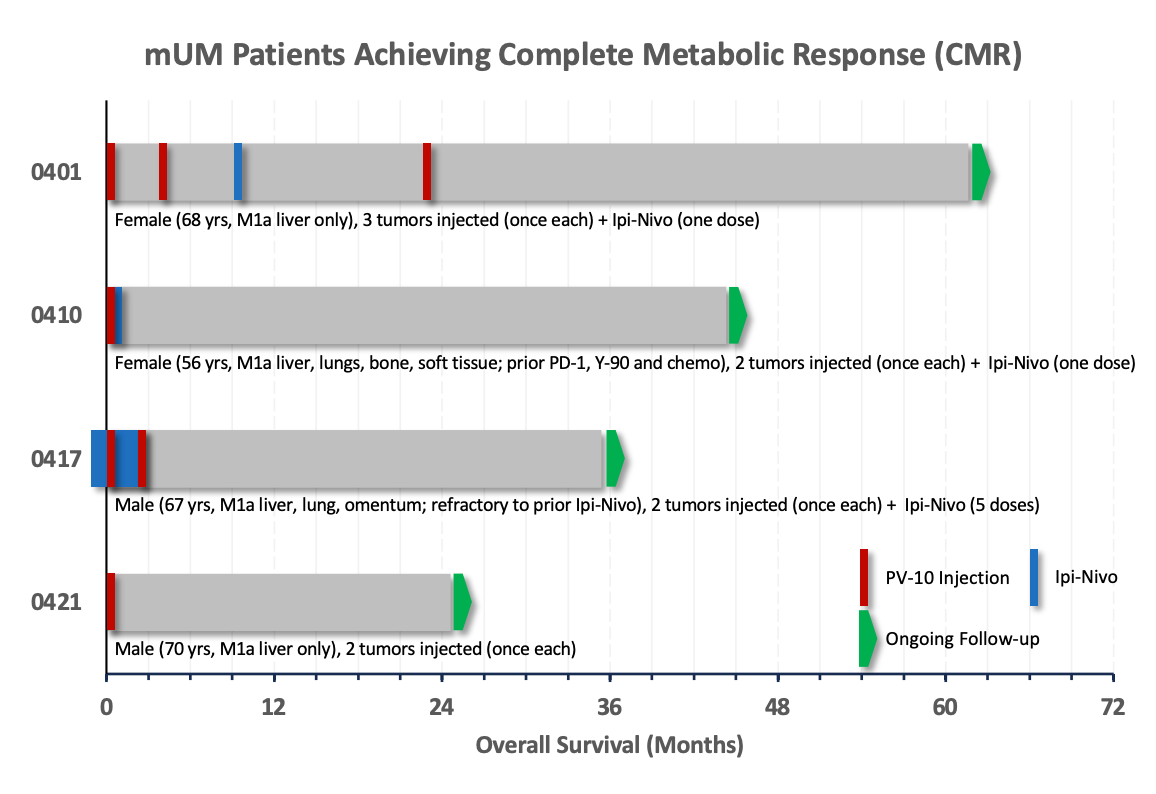
Both SMR poster presentations will report updated overall survival (OS) data.
One of the main criticisms that a regulatory consultant voiced to us in 2018 (i.e., an aspect of our review-and-analysis efforts after securing control of Provectus’s board of directors in 2017) was the lack of durability-of-response data. This deficit can be addressed by, specifically, progression-free survival (PFS) and, generally, overall survival (OS), where the former can be used by regulators and industry as a surrogate endpoint for the latter. Durability and survival, if they occur, take time.
Provectus’s Phase 2 clinical trial of single-agent PV-10 for the treatment of metastatic melanoma did not formally (i.e., within the study protocol) collect durability or survival data beyond 12 months of patient follow-up post-PV-10-treatment (see “Chemoablation of Metastatic Melanoma with Rose Bengal (PV-10),” 2010 American Society of Clinical Oncology (ASCO) annual meeting.
Provectus’s emerging hepatic tumor data, which Ed Pershing queried then-management strenuously, regularly, and repeatedly about for many years prior to 2017:
First showed informally here in April 2015, see They Live (April 11, 2015), and “Oops” (April 10, 2015), and Initial Clinical Testing: HCC and Liver Mets (April 10, 2015): 7 out of 10 patients with hepatic tumors treated with PV-10 were alive after up to 50 months (i.e., >4 years),
Showed formally in July 2015, “Phase 1 Study of PV-10 for Chemoablation of Hepatocellular Cancer and Cancer Metastatic to the Liver”, European Society for Medical Oncology (ESMO) World Congress on Gastrointestinal Cancer: 10 out of 13 patients were alive after up to 54 months (~4.5 years),
We pushed for a comprehensive analysis of the hepatic tumors program (another aspect of our review-and-analysis efforts) that resulted in Provectus’s April 2020 poster presentation “Oncolytic immunotherapy of hepatic tumors with intralesional rose bengal disodium” in the ePoster Library of the Society of Interventional Radiology (SIR). Notably:
8 types of hepatic tumors (HCC, colorectal, uveal melanoma, lung, cutaneous melanoma, breast, ovarian, and pancreatic) were treated with single-agent PV-10 and/or PV-10-led combination therapy,
Median disease-specific survival (mDSS) was not reached for HCC patients (range 0.1 months to 9+ years), and
Median disease-specific survival (mDSS) was 26.8 months (>2 years) for metastatic colorectal patients (range 0.1 months to 8+ years).
At Provectus’s 2023 annual stockholder meeting, we presented data (see slide #12) of a patient from the hepatic tumor program who received a single injection of PV-10 to one of two pancreatic tumors, declined to receive chemotherapy after 17 months (~1.5 years), and survived for 29 months (~2.5 years) in total.
We also ran Provectus’s clinical trial of the combination therapy of PV-10 and pembrolizumab for three distinct patient cohorts — checkpoint-naive and checkpoint-refractory Stage IV cutaneous melanoma, and checkpoint-naive Stage III melanoma — to explicitly collect durability-of-response and survival data, not merely patient (and PV-10-injected lesion) response data.
We approached Provectus’s work in mUM in the same fashion. Longer OS provided us the opportunity to reflect on disease response; that is, metabolic complete response (mCR), also known as complete metabolic response (CMR) and measured by Positron Emission Tomography Response Criteria In Solid Tumors (PERCIST). This first emerged in our mUM clinical study, and is applicable to our ongoing neuroendocrine tumor (NET) metastatic to the liver (mNET) and contemplated pancreatic cancer metastatic to the liver studies.
As an aside, consider 2016 article “Use of PERCIST for Prediction of Progression-Free and Overall Survival After Radioembolization for Liver Metastases from Pancreatic Cancer:”
CONCLUSION
Metabolic response by PERCIST predicts OS, PFS, and TTP after radioembolization for liver metastases from pancreatic cancer. Our findings underline the feasibility of 18F-FDG PET/CT with PERCIST-based response prediction for local treatment of liver metastases from pancreatic cancer and may also generalize to liver metastases of nonpancreatic origin.
Several germane article references include: “F-FDG proved to be predictive of response to radioembolization:”
6. Haug AR, Heinemann V, Bruns CJ, et al. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging. 2011;38:1037–1045.
7. Haug AR, Tiega Donfack BP, Trumm C, et al. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53:371–377.
8. Fendler WP, Philippe Tiega DB, Ilhan H, et al. Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J Nucl Med. 2013;54:1202–1208.
9. Gulec SA, Suthar RR, Barot TC, Pennington K. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing 90Y selective internal radiation therapy plus chemotherapy. Eur J Nucl Med Mol Imaging. 2011;38:1289–1295.
10. Soydal C, Kucuk ON, Gecim EI, Bilgic S, Elhan AH. The prognostic value of quantitative parameters of 18F-FDG PET/CT in the evaluation of response to internal radiation therapy with yttrium-90 in patients with liver metastases of colorectal cancer. Nucl Med Commun. 2013;34:501–506.
What other therapies display the potential for a patient to achieve CMR (not just metabolic response)?
More successful response to vaccination
The SITC poster presentation is an offshoot to Dr. Narendran’s lab team’s preclinical work on the involvement of the stimulator of interferon (IFN) genes (STING), a central innate immunity pathway, in PV-10-mediated immune activation. Dr. Narendran’s initial STING results were presented in June 2020 on a poster at the American Association for Cancer Research (AACR) 2020 Virtual Annual Meeting II.
STING is an important pathway in cancer, such as pancreatic cancer.
We provided a further update on this work in January 2022 when we disclosed a patent application made by Provectus entitled “Halogenated Xanthenes as Vaccine Adjuvants,” when the application was published on the U.S. Patent and Trademark Office’s (USPTO’s) website.
Some context: An example of a commercial-stage vaccine adjuvant is Dynavax Technologies’s CpG 1018, which is based on Dynavax’s proprietary toll-like receptor 9 (TLR9) agonist. Dynavax attempted to create a biologic-based TLR9 agonist intralesional (IL) therapy, which Dynavax then sold to TriSalus Life Sciences, which is trying to infuse (i.e., a different route of administration from IL injection) the agent for the treatment of solid tumor cancers. Another TLR9 IL cancer agent is vidutolimod, which was initially developed by Checkmate Pharmaceuticals before the company was acquired by Regeneron Pharmaceuticals.
We provided an update in October 2021 on Provectus’s work at the intersection of cancer and virology, when we noted the USPTO publication of Provectus’s patent application entitled “Novel Uses of Halogenated Xanthenes in Oncology and Virology.”
The upshot here is that we believe — at the risk of comparing apples (synthetic small molecule RBS) and oranges (other biologics, whether small or macro molecules) — that RBS potentially has a constructive, stronger, more relevant, more precise, longer-lasting, etc. impact on the immune system, as a cancer agent or as an added component of a vaccine.