Provectus Files Form 10-Q for the Fiscal Quarter Ended September 30, 2023
Financial Results (unaudited) through September 30, 2023 (3Q23).
Earlier today, Provectus filed its Form 10-Q for the fiscal periods (three and nine months) ended September 30, 2023.
Footnotes.
Cover page; Notes 3 (Significant Accounting Policies) and 8 (Stockholders’ Deficit).
Fully diluted shares outstanding. The Company noted in its Form 8-K filing on September 7, 2021 that Provectus’s fully diluted shares of common stock outstanding in early-2017 was approximately 564 million on an as converted basis, consisting of common stock, private and publicly traded warrants, stock options, and then-Series B preferred shares.
After consideration of the $29.2 million raised as Series D and Series D-1 preferred stocks from 2017 to date, as well as accrued interest on the preferred stocks’ initial convertible notes, the Company’s fully diluted shares of common stock outstanding as of September 30, 2023 was approximately 548 million on an as converted basis, consisting of common stock, private warrants, stock options, and Series D/D-1 preferred shares.
Notes 11 (License Transactions).
Bascom Palmer Eye Institute (BPEI). The University of Miami’s (UM’s) Office of Technology Transfer (OTT) asked Provectus to transition term sheet discussions (and a then-near-final, non-binding term sheet) to a draft full license agreement, which OTT and the Company are endeavoring to finalize. In May 2023, we provided OTT with a preliminary development plan for potentially commercializing rose bengal sodium (RBS) photodynamic antimicrobial therapy (PDAT) in ophthalmology.
Should Provectus consummate an agreement with OTT, we would further discuss BPEI’s intellectual property, the potential addressable market opportunity in ophthalmology for RBS PDAT, potential business strategy(ies), and other topics in a future Provectus Substack post. The Company may also contemplate an exclusive worldwide license from UM to Provectus that potentially could be exclusively sub-licensed by a Provectus spun-out start-up company focused on the field of ophthalmology.
MD&A.
Clinical Development and Drug Discovery.
Penile SCC. An academic medical center approached Provectus to discuss potentially initiating an investigator-initiated study (ISS; i.e., a Phase 1 clinical trial) of PV-10 for the pre-operative treatment of penile squamous cell carcinoma (SCC; PSCC). The medical center previously had completed in vivo and in vitro work, and plans to pay for the IIS. Penile cancer is a rare disease, with an incidence of less than one per 100,000 men and a prevalence in the U.S. of 2,000-2,5000 new cases and 400-500 deaths per year.
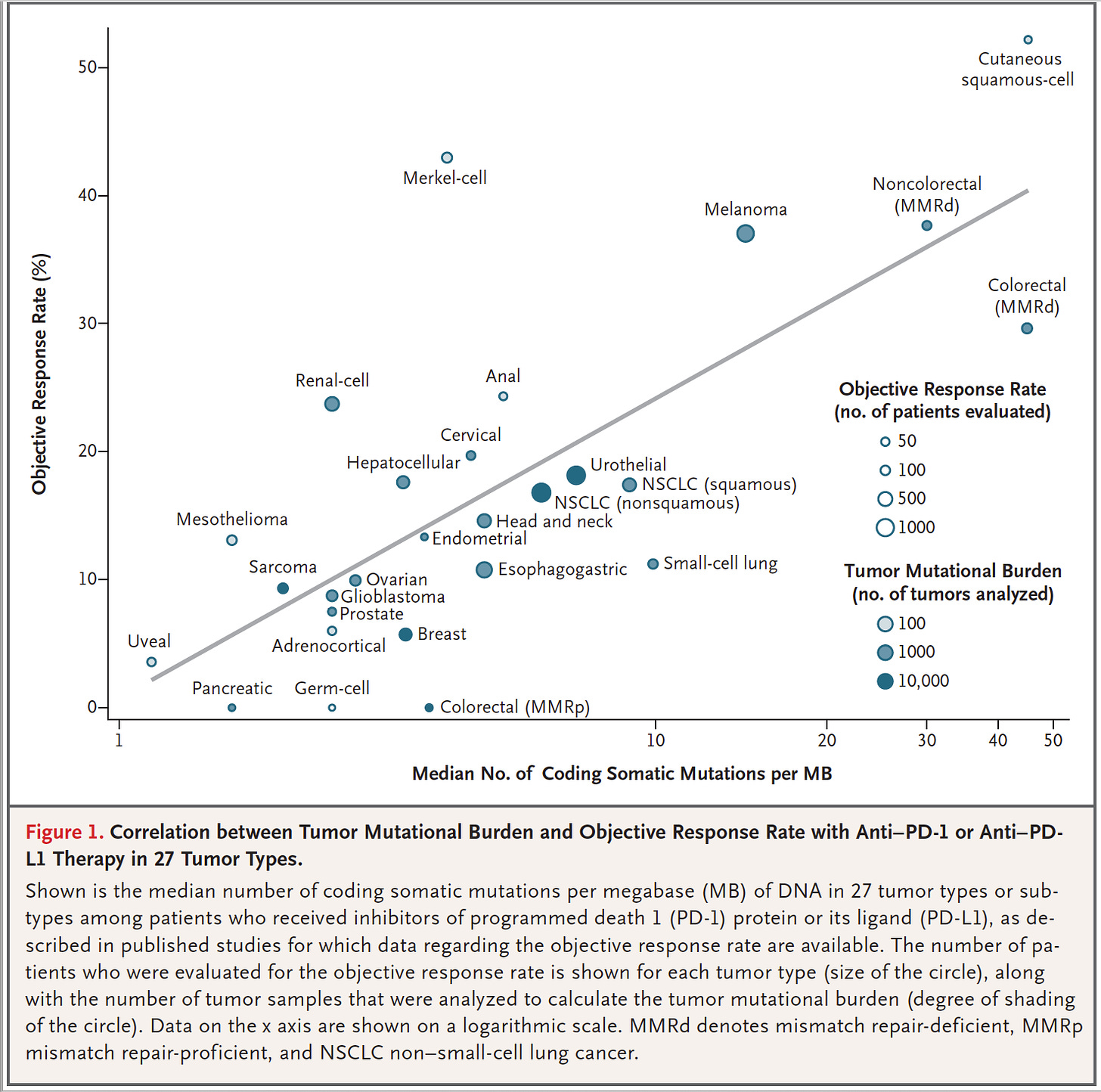
Referencing Figure 1 of Yarchoan et al. 2017 (Tumor Mutational Burden and Response Rate to PD-1 Inhibition), penile PSCC may range from (a) the lower left-hand corner of the figure (i.e, low tumor mutational burden [TMB] and low checkpoint response), and could be closer in “tumor coldness” to uveal melanoma and pancreatic cancer1, to (b) being similar in make-up to head and neck SCC (HNSCC) and non-small cell lung cancer (NSCLC) (squamous)23.
Checkpoints do not appear to work for PSCC (i.e., no drug approvals as yet), and may be recommended for penile cancer with higher TMB (i.e., closer to HNSCC and NSCLC), which are about 18% of PSCC patients4.
Forward-Looking Statements
The information in Provectus’ Substack post may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in Provectus’ Substack are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, and
Provectus’ Quarterly Report on Form 10-Q for the period ended September 30, 2023.