Provectus’s PV-10 (rose bengal sodium) as a vaccine adjuvant
SITC 2023.
On Monday (November 6, 2023), Provectus press released a poster from the Society for Immunotherapy of Cancer (SITC) 2023 annual meeting, which was held from November 1-5 in San Diego, California. The poster was prepared by Aru Narendran, MD, PhD and his lab team from the Cumming School of Medicine at the University of Calgary in Alberta, Canada. Its subject was ongoing research on the potential use of investigational cancer immunotherapy PV-10 (drug substance: rose bengal sodium, or RBS) as an adjuvant in vaccines to help them work better.
A copy of the SITC poster, titled The iodinated fluorescein derivative PV-10 enhances the antiviral activity of CD8+ T-Cells by inducing STING dimerization: Implications for enhanced vaccine applications, is available on Provectus’s website at https://www.provectusbio.com/media/docs/2023-SITC-poster.pdf.
The Narendran lab has advanced the concept of PV-10 as a vaccine adjuvant for the first time in the formal, medical conference setting of SITC, and the prospects of PV-10 potentially contributing to greater protection against disease by improving a person’s immune response to vaccination.
Dr. Narendran and his colleagues’ work has spanned several years, including but not limited to milestones of (i) the Narendran lab’s initial discovery about PV-10’s unique modulation of the STING pathway (2020), a consequential innate signaling pathway, (ii) Provectus’s patent application for RBS as a vaccine adjuvant (2022), (iii) Dr. Narendran’s formal and further data on PV-10’s vaccine adjuvant-edness (2023), and (iv) manuscript for publishing being developed (ongoing).1
As noted above, Provectus initially introduced the vaccine adjuvant concept (utilizing early Nanrendran lab data) when it disclosed that the Company’s patent application on the topic was published on the U.S. Patent and Trademark Office’s (PTO’s) website in January 2022: Halogenated Xanthenes as Immune Adjuvants (application no. 17/488,430). We also press released this.
The importance of a vaccine adjuvant.
An adjuvant is a compound added to a vaccine that can enhance the immune response to a target antigen or target antigens, which is (are) the payload(s) of the vaccine. Some antigens cannot generate a strong enough immune response by themselves. The antigen payload is representative of the target disease against which vaccination is sought.
Vaccine adjuvants are important for other reasons too, including but not limited to: reducing the amount of antigen needed, which may be more cost-effective and reduce adverse reactions; extending the duration of immunity provided by a vaccine, which may mean, among other things, fewer booster shots over time to maintain protection; broadening the range of vaccine targets (i.e., expanding the number of diseases that could be effectively targeted by vaccines); and improving vaccine effectiveness in specific populations (e.g., individuals with weakened or compromised immune systems).
PV-10 research.
We have written before on Provectus’s Substack about the impact of the coronavirus pandemic on the Company’s and its collaborators’ research, including strategy, directions, and outcomes; see The Impact of COVID-19 on Provectus’s Research & Development, Halogenated Xanthene Medical Science Platform, and Rose Bengal Sodium-based Investigational Drug & Drug Formulation Pipeline (April 7, 2023).
Under a Covid-19 timeline of late-2019 to mid-2022, Dr. Narendran let us know in mid-2020 (i.e., about a third of the way through this pandemic timeline) of his progress testing the hypothesis that PV-10 could function as a vaccine adjuvant. His lab team’s and his path of investigation followed directly from his team’s discovery of PV-10’s unique modulation of stimulator of interferon (IFN) genes (STING), which they presented as a poster at the AACR 2020 Virtual Annual Meeting II. Again, STING is one of the most important innate signaling pathways.
Selective multiplicity.
Provectus’s initially mechanism or targeting work centered around PV-10’s lysosomal targeting, and thus lysosome-dependent cell death (LDCD). Company collaborators – three of them: Pilon-Thomas (from Moffitt Cancer Center), Maker (then from the University of Illinois at Chicago, now at the University of California, San Francisco), and Narendran have all implicated immunogenic cell death (ICD) from PV-10 treatment. Narendran has also shown that PV-10, in addition to being stimulatory, has several inhibitory mechanisms or targets.
So, it is not so much that PV-10 also happens to be, among other things, a STING agonist (i.e., PV-10 and, more specifically, RBS are selectively multiplicitous). Rather, Dr. Narendran and his lab team’s work was novel in so far as they identified that PV-10 uniquely activated STING; that is, the pathway’s activation differed from the activation by other STING agonists/agents that have mostly if not exclusively failed or underwhelmed in the clinic.
The larger point here is that we keep seeing RBS’s selective multiplicity – the targeting and signaling of several things in different (than the “norm”) ways; not one thing in one way – in the preclinical and clinical data that Provectus has generated and continues to generate.
Repeatability.
Eleven years ago at the SITC 2012 annual meeting, Provectus’s original scientific founders initially advanced the concept of intratumoral injection of PV-10 into cancer tumors and lesions as tantamount to in situ vaccination; see the Company’s poster titled Generation of an Antitumor Response and Immunity Using a Small Molecule Drug (PV-10). Moffitt Cancer Center’s poster presentation at the AACR 2013 annual meeting six months later, titled Intralesional Injection with PV-10 Induces a Systemic Anti-tumor Immune Response in Murine Models of Breast Cancer and Melanoma, validated some of Provectus’s assertions.
The Narendran lab, at SITC 2023, at AACR 2020, and in ongoing research, have importantly validated and consequentially expanded upon the Company’s original claims – now in both virology and oncology.
Vaccine adjuvant, vaccine, or both?
Based on Provectus’s preclinical and clinical data to date, we believe that the response to PV-10 treatment for cancer is very much tantamount to in situ vaccination. It would then seem possible that PV-10-adjuvanted vaccines could potentially contribute to more effective and durable immune responses, better responses from patient populations with unique characteristics, higher efficacy using less antigen, and making vaccines more accessible, sustainable, and affordable.
The same Provectus SITC 2012 poster also importantly noted (about in vivo [i.e., murine model] work) that PV-10 treatment for cancer is capable of tumor-specificity (i.e., disease-specificity), long-term immunity (in context), and adoptive transfer of immunity.
Repeatability redux.
The Narendran lab also validated Provectus’s prior scientific founders’ assertions related to PV-10’s LDCD, which was not a thing in 2002 when the Company’s co-founder Eric Wachter, Ph.D. published his visuals of LDCD of adult hepatocellular carcinoma and breast cancer cells.
Dr. Narendran and his lab team members demonstrated LDCD in [pediatric] neuroblastoma cells, publishing their results in a 2019 paper titled Potent in vitro and xenograft antitumor activity of a novel agent, PV-10, against relapsed and refractory neuroblastoma.
The chicken or the leg.
A vaccine adjuvant is supposed to elicit a more potent immune response when added to the antigen in a vaccine because, mechanistically, the adjuvant boosts the immune system’s response to the antigen.
Take the example posed in the illustration below.
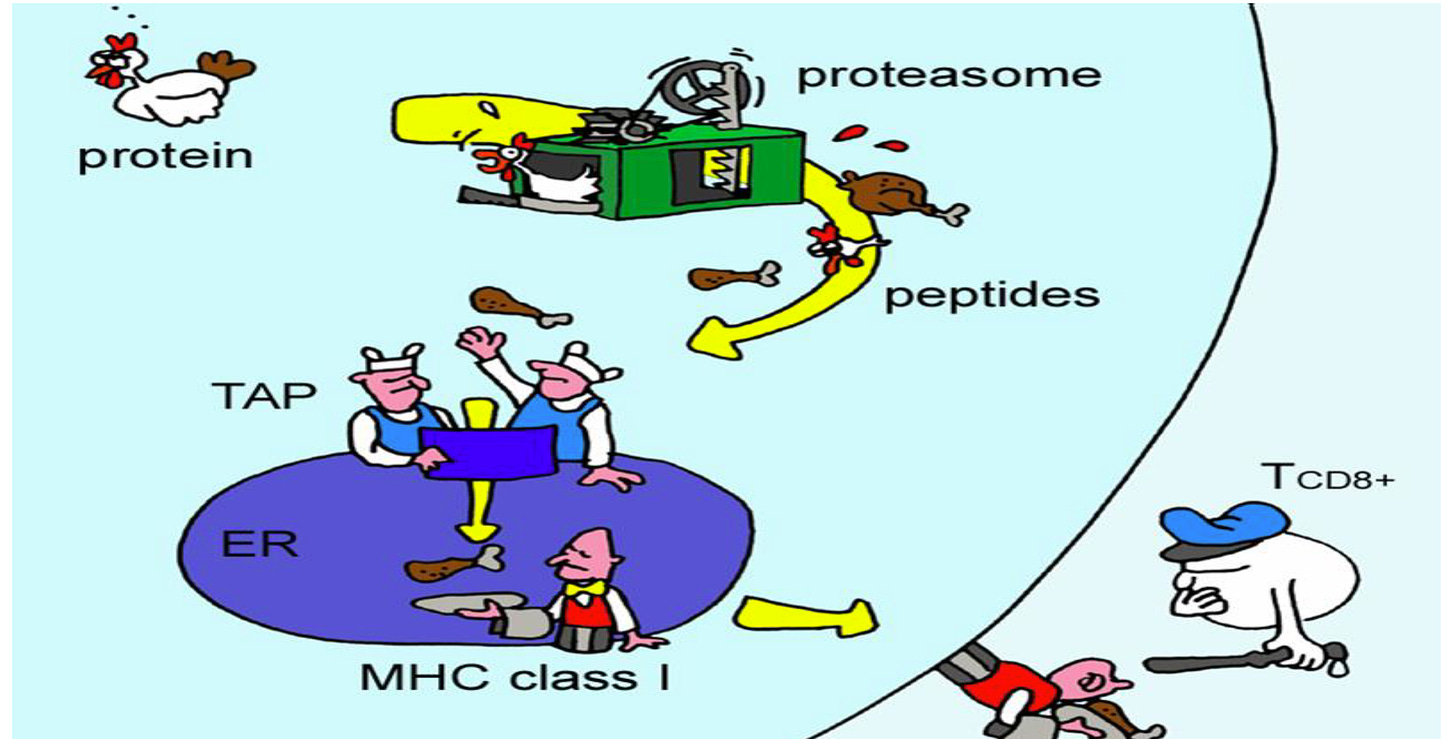
The goal is to get the adaptive immune system (i.e., TCD8+) to recognize the target disease (e.g., a tumor, a pathogen, etc.) by target antigenic protein(s) (i.e., the chicken).
As you may note in the cartoon, this approach process the chicken, eventually presenting part of it (e.g., the disembodied leg absent feathers and the rest of the chicken) to the immune system. Under this approach, the immune system is trained to identify a very simplistic representation of the antigen payload (of the target tumor or pathogen).
PV-10 may work as well as it does, insofar as the clinical data we have presented may show, in part, because it helps to avoid the processing and refining steps (of innate immune system signaling) and allow the immune system to directly recognize the chicken. In other words, the target protein of the target disease is the chicken, and the chicken in all (or nearly all of) its form, function, and detail is presented to the [adaptive] immune system.
Questions may remain about the exact mechanism(s) of an adjuvant(s) (e.g., Awate et al. 2013, Zhao et al. 2023); however, the potency of an adjuvant’s innate immune system signaling, leading to the strength of the adaptive immune system’s response, may be key.
We are very excited by Dr. Narendran and his lab team’s data and evidence thus far because we believe that Provectus can directly tie the potential for PV-10 as a vaccine adjuvant to the innate signaling that comes from PV-10 treatment for cancer (as well as the potential for PV-10 as both a monotherapy and combination therapy for injectable solid tumor cancers), which Provectus has shown through clinical data. We wrote about innate signaling in Provectus’s Substack titled Cancer Immunotherapy PV-10's Innate Immune Signaling (June 3, 2023).
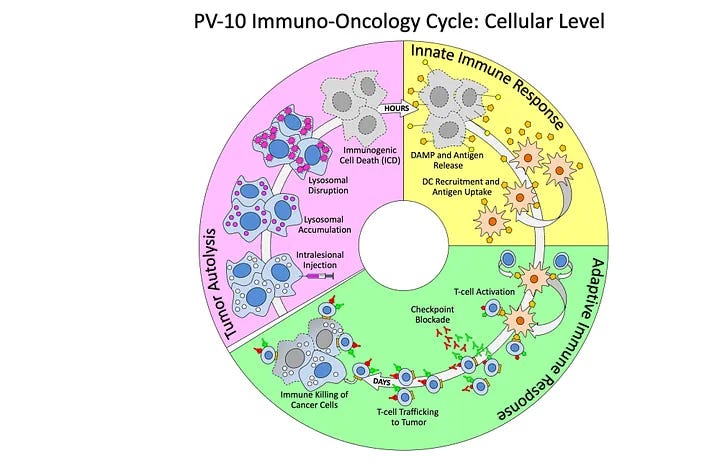
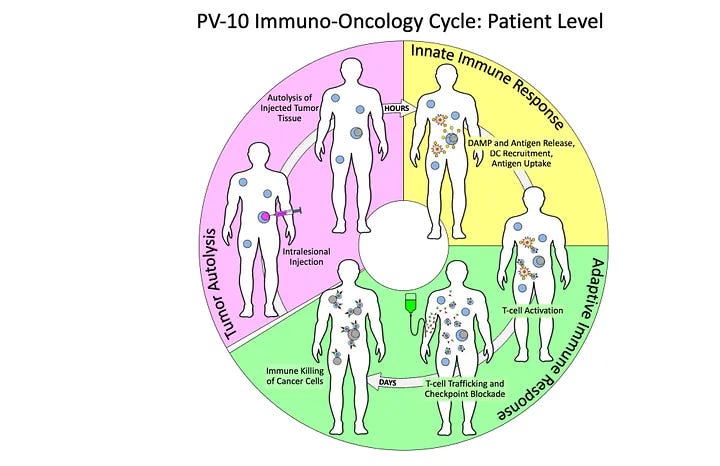
In cancer, Provectus has shown preclinically and clinically PV-10’s three-step, multi-variate, interconnected and interrelated systemic mechanism:
· Within hours of PV-10-injection: tumor tissue cell death,
· Innate immune signaling from the release of DAMPs, tumor antigens, cytokines, etc. from PV-10-injected tumors, and
· Within days of PV-10 injection: Tumor-specific functional T cell response.
Another conclusion.
In cancer treatment, real, proper, and effective innate signaling is important and appropriate to keep in mind when considering various immunotherapy combination approaches, because the immune system response that PV-10 itself generates from tumor treatment (i.e., the adaptive response that emanates from innate signaling that comes from proper cell death that comes from PV-10 treatment of drug agent interacting directly with disease) can be boosted, expanded, “loudened,” etc. by a drug partner (such as an immune checkpoint inhibitor, or perhaps even chemotherapy). Thus, the richness of innate signaling is key to the success of pairing beyond the monotherapy efficacy of the drug partner.
The intersection of virology and oncology.
Provectus disclosed that the Company’s patent application on the intersection was published on the PTO’s website in September 2021: Novel Uses of Halogenated Xanthenes in Oncology and Virology (application no . 17/212,723).
In the first paragraph under Background Art of the application, we wrote “[o]ncology and virology are tangentially related fields that intersect at the innate and adaptive immune systems of animals, in particular humans. Whereas disease etiology and manifestations are generally distinct, this intersection provides a common basis for the application of discoveries in one field to the other.”
Some implications.
Dr. Narendran and his lab team’s discovery of PV-10’s potential as a vaccine adjuvant fits rationally and scientifically supportively within interrelated and interconnected mechanisms of PV-10. In other words, their work is consistent with Provectus’s, theirs, and others’ work on RBS.
The discovery provides yet another opportunity to expand Provectus’s medical science platform and broaden its range of uses, particularly some rather consequential ones.
Forward-Looking Statements
The information in Provectus’ Substack post may include “forward-looking statements,” within the meaning of U.S. securities legislation, relating to the business of Provectus and its affiliates, which are based on the opinions and estimates of Company management and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements. Forward-looking statements are often, but not always, identified by the use of words such as “seek,” “anticipate,” “budget,” “plan,” “continue,” “estimate,” “expect,” “forecast,” “may,” “will,” “project,” “predict,” “potential,” “targeting,” “intend,” “could,” “might,” “should,” “believe,” and similar words suggesting future outcomes or statements regarding an outlook.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated or that such agents as products will achieve any particular revenue levels.
Due to the risks, uncertainties, and assumptions inherent in forward-looking statements, readers should not place undue reliance on these forward-looking statements. The forward-looking statements contained in Provectus’ Substack are made as of the date hereof or as of the date specifically specified herein, and Provectus undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except in accordance with applicable securities laws. The forward-looking statements are expressly qualified by this cautionary statement.
Risks, uncertainties, and assumptions include those discussed in the Company’s filings with the Securities and Exchange Commission (SEC), including those described in Item 1A of:
the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, and
Provectus’ Quarterly Report on Form 10-Q for the period ended June 30, 2023.
For Provectus’s business development activities, it is helpful and seemingly necessary to have such data and information in the public domain, typically in medical conference presentations and peer-reviewed medical journals, so that the material is then non-confidential and can thus be shared readily and without the immediate need for a confidential or non-disclosure agreement (CDA/NDA).



